SENSe: Assessing neurorehabilitation’s impact on the somatosensory function in stroke patients

Authors: Irina Benedek, Oana Vanta
Keywords: stroke rehabilitation, somatosensory disorders, upper extremity, clinical trial
The impact of stroke on somatosensory function in stroke patients
Between 50% and 85% of stroke patients are negatively impacted by the loss of bodily sensations like touch discrimination [1]. Therefore, the person’s capacity to explore his local environment and carry out daily activities such as gripping and moving things is impaired. Touch discrimination also decreases personal safety and quality of life and consequently lengthens hospital stays. However, in hospitals, sensory loss is frequently disregarded. Clinicians either fail to address the issue or use approaches with weak theoretical or empirical foundations [2].
Not many retraining techniques concentrate on persistent improvement and sensory discrimination, even though somatosensory stimulation protocols have been carefully examined; they were mainly assessed concerning motor recovery and temporary effects [3].
Carey and the team [2] used a randomized controlled trial to assess the efficacy of the developed method for teaching sensory discrimination in the upper limb. The control intervention consisted of repeated exposure to sensory stimuli in stroke patients with sensory impairment. In contrast to the control intervention, the authors hypothesized that stroke survivors who received the discrimination training would show a more significant gain in their ability to distinguish between different sensory inputs, with maintenance at follow-up.
Carey et al. chose the most effective training strategy, generalized enhanced sensory training, intending to help stroke patients relearn basic sensory modalities like texture discrimination, limb position perception, and object recognition by touch [2].
Study addressability
From the 50 stroke survivors enlisted, twenty-five were randomly assigned to the experimental intervention and 25 to the control intervention. The patient eligibility criteria are described in Figure 1 below.
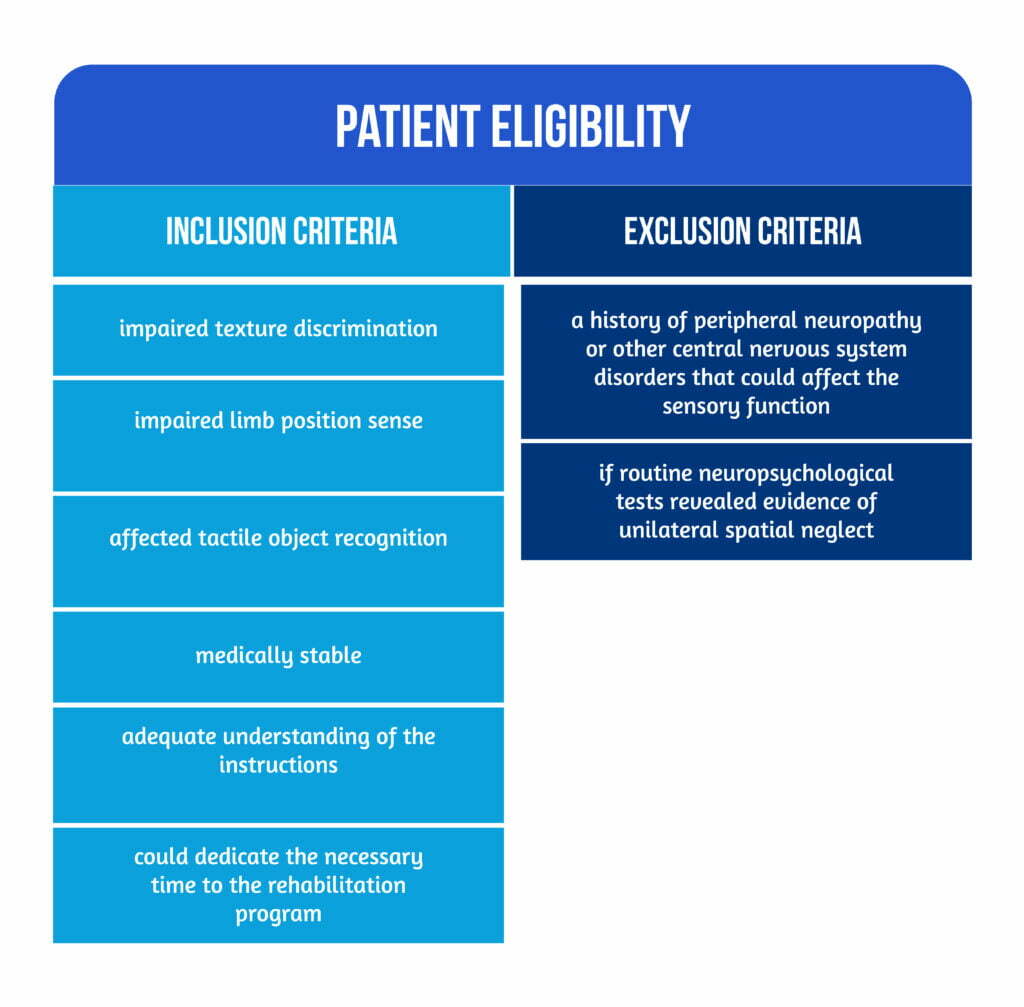
Patients were chosen from six participating hospitals in Melbourne’s metropolitan area. Long-term community-based facilities connected to the hospitals were also included. To reduce potential confounding from co-therapies, patients were enrolled in the trial only after completing their inpatient and outpatient therapy or community-based follow-up [2].
Interventions
- The experimental intervention (EI): used texture discrimination, limb position sense, and tactile object recognition as three sensory tasks to apply the generalized sensory discrimination training principles [4]. Different stimuli were used in each sensory dimension during training, and there was a graded development of discriminations from simple to complicated as well as anticipation trials, focused exploration with vision occluded, and patient feedback. A wide variety of upper limb positions were used to develop limb position perception. At the same time, tactile object recognition training focused on distinguishing the shape, size, weight, texture, hardness, and temperature of different objects [2].
- The control intervention (CI): consisted of repetitive non-specific exposure to stimuli that varied in texture, shape, size, weight, hardness, and warmth through the passive use of the upper limb and the grabbing of everyday objects. Exposure was chosen as the CI because it is analogous to experience in everyday activities and therapy, the current defining experience, and because former studies have shown that most stroke survivors do not improve with repeated exposure alone [4, 5].
Study Design – randomization and evaluation patterns
A randomized prospective parallel-group controlled study was carried out, including within- and between-subject experimental controls. The primary outcome was to evaluate the difference in sensory discrimination ability across groups at the end of phase 1 (after sensory discrimination training). In phase 2, the experimental group had a second round of sensory training while the control group underwent sensory discrimination training in a cross-over arm. This strategy had the following advantages:
- used fewer resources,
- enabled research into the additional advantages of sensory training above the existing exposure-only strategy with the subject acting as their own control
- enabled examination of treatment dosage [2].
Computer-generated randomization used proportionate sampling to account for gender and side of the lesion. The allocation for the intervention was kept from the recruiting therapists. Independent assignment was centrally supervised by a researcher who had no direct interaction with stroke patients and only distant communication with the treating therapists to send them sealed opaque envelopes or electronic mail informing them of the randomization.
The first right-sided lesion subject to arrive was randomly assigned to the experimental or control group. The second right-sided lesion patient (of the same gender) was randomly assigned to the opposite group. Every subsequent pair of right- or left-sided lesion participants underwent the same procedure, with the first member always randomly chosen [2].
A phase was made up of 10 intervention sessions that were held three times a week for around 60 minutes each. For outcome assessment, the evaluations were conducted as follows:
- before phase 1 (A0)
- at the end of phase 1 following experimental or control intervention (A1)
- both the immediate and delayed intervention groups at the end of phase 2 following experimental intervention (A2)
- six weeks (A3) and six months (A4) post intervention [2].
Participants were requested not to change their usual pattern of exposure to sensory stimuli throughout the study and were not allowed to participate in any other sensory retraining.
Several parameters were assessed such as demographic data, stroke type, severity, and clinical impact, as well as the somatosensory impairment through touch detection, texture discrimination, limb position sense, temperature discrimination, and tactile object recognition. A composite index of functional somatosensory discrimination ability was used as the primary outcome measure. It was created by combining standardized indexes for texture discrimination, limb position perception, and tactile object identification. To increase the sensory function across three modalities, the training program’s design called for a composite measure [2].
The sensory program was clinically oriented and designed to retrain 3 sensory discrimination functions, as described in Figure 2 below.
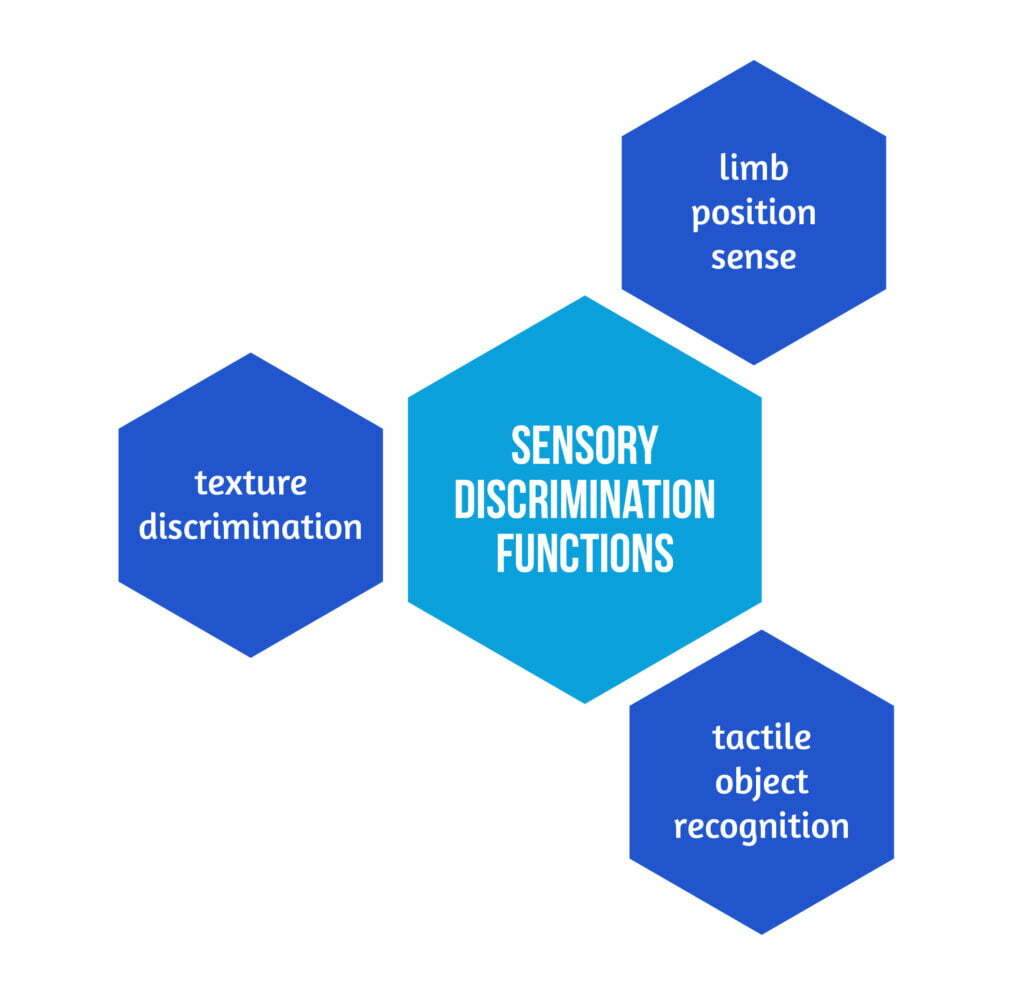
These abilities were selected as they affect function in their own right. The available time to train each modality was significantly shorter than in earlier experiments, in which only one modality was treated at a time, as they were addressed concurrently [2].
Results – essential data with a clinical impact
The groups’ initial baseline demographic and clinical features were comparable. The average age of the group was 61.02 years. There were a total of 29 left and 21 right hemispheric lesions. Moreover, there were no between-group differences at baseline in the Standardized Somatosensory Deficit (SSD) scale, time poststroke, National Institutes of Health Stroke Scale score (NIHSS), or lesion side [2].
Throughout the five-time points, the immediate and delayed intervention groups demonstrated progress in the anticipated direction for the primary outcome.
🡪 For individuals who received the EI, phase 1 change analysis showed a significant improvement in SSD.
🡪 The CI group, in contrast, had a significantly lower average change.
The anticipated comparison proved that the EI group considerably improved sensory discrimination compared to the CI group.
The delayed intervention group had an average improvement at phase 2 (EI) in SSD that was greater than in phase 1. However, this improved deficit reduction under EI did not reach statistical significance. A closer look at the amount and direction of change during phase 1 indicated that the EI group had improved more than the CI group had. Most of the EI group (22/25) showed improvement over baseline. Both groups demonstrated a substantial intervention impact at the 6-week and 6-month follow-ups compared to preintervention status. At six weeks, all but one person showed an improvement [2].
As a result of the specific design assessing the 3 sensory discrimination functions and the timeframe for completing each training, it was not unexpected that the amplitude of the change seemed to be somewhat smaller than when only one training modality was used. In such investigations, a higher percentage of participants received results within the normal training modality range [2].
Nevertheless, the encouraging results offer a solid basis for dose-response studies that aim to tailor training settings for specific populations.
Conclusions
The results of the above-presented research support the inclusion of sensory discrimination training in the rehabilitation of stroke survivors with sensory impairment. Patients had been recovering from stroke for at least 6 weeks; therefore, they were in the chronic stage of rehabilitation [2].
Carey and the team demonstrated the therapeutic utility of sensory retraining by assessing the quantification of training effects and maintenance of gains at short- and long-term follow-ups.
Therapists may apply the method in both home and rehabilitation settings since it tries to enhance lost abilities rather than place a heavy emphasis on compensating.
Given the high incidence of sensory loss and its detrimental impact on effective environment exploration and daily activity performance, this is an important outcome for stroke therapy. Assessing the percentage reduction in the sensory deficit and preservation of improvement at 6-week and 6-month follow-ups also demonstrates the clinical importance of the intervention effect [2].
To learn more about neurorehabilitation, visit:
- Can gaming therapy impact post-stroke rehabilitation?
- Obesity role in stroke prognosis and rehabilitation
- Rehabilitation of non-fluent aphasia patients
References
- Carey LM. Somatosensory loss after stroke. Crit Rev Phys Rehabil Med. 1995;7:51-91. Available at: https://www.dl.begellhouse.com/journals/757fcb0219d89390,58e9038f7231b7dc,7af41ca30975d27f.html
- Carey L, Macdonell R, Matyas TA. SENSe: Study of the Effectiveness of Neurorehabilitation on Sensation: a randomized controlled trial. Neurorehabil Neural Repair. 2011;25(4):304-13. doi: 10.1177/1545968310397705.
- Carey LM, Blennerhassett J, Matyas T. Evidence for the retraining of sensation after stroke remains limited. Critically appraised papers. Commentary. Aust Occup Ther J. 2010;57:200-202. DOI: 10.1111/j.1440-1630.2010.00867.x
- Carey LM, Matyas TA. Training of somatosensory discrimination after stroke: Facilitation of stimulus generalization. Am J Phys Med Rehabil. 2005;84:428-442. DOI: 10.1097/01.phm.0000159971.12096.7f
- Carey LM, Matyas TA, Oke LE. Sensory loss in stroke patients: Effective training of tactile and proprioceptive discrimination. Arch Phys Med Rehabil. 1993;74:602-611. DOI: 10.1016/0003-9993(93)90158-7