The feasibility of repetitive sensory stimulation in the rehabilitation of the upper limb after a stroke (the PULSE-I study)
Authors: Ioana Stanescu, Irina Benedek, Oana Vanta
Keywords: sensory stimulation, stroke rehabilitation, feasibility study
The burden of stroke
Why tackle the feasibility of repetitive sensory stimulation in the rehabilitation of the upper limb after a stroke? More than 15 million people suffer from stroke each year, representing the leading cause of acquired disability in developed countries [1]. Despite recent advances in the acute care of cerebrovascular accidents, the rehabilitation of sensorimotor function of the upper limb remains a challenge; less than 20% of stroke patients regained full function of the upper limbs, and more than half did not recover essential function after a few years [2, 3]. Consequently, the quality of life is affected due to the inability to perform daily activities that involve the upper limbs. Therefore, regaining function at this level is critical for stroke neurorehabilitation programs.
Learn more about the recovery of precise hand movements after stroke.
The need for innovations in neurorehabilitation programs
Current guidelines support the concept of repetitive task training (RTT), which refers to a high number of motor task repetitions to enhance sensorimotor function [4]. Since neuroplasticity occurs mainly in the first 5 weeks after stroke, this interval should be used to maximize and adapt recovery programs for the upper limb [5]. However, high-intensity RTT in the early phase after a cerebrovascular accident is challenging in clinical practice. It demands patients remain consistent and motivated while the medical staff should be available and have the required resources [6, 7].
Accordingly, developing and implementing new treatment programs that aim to rehabilitate the upper limb is urgent. The main goals of such programs are
- The ability to be implemented in the early phase after a stroke
- Not require extra time from the specialized staff
- Not rely entirely on the patients’ motivation after stroke
- The ability to be used by patients with severe impairments
Repetitive sensory stimulation for upper limb sensorimotor function recovery
Repetitive sensory stimulation (RSS) is a primarily passive treatment that determines neuroplastic changes in the general healthy population, similar to repetitive task training [8]. RSS has been assessed in studies of chronic stroke patients months or even years after a cerebrovascular accident. Positive upper limb function and sensation results, including tactile discrimination, were obtained [9]. It has been demonstrated that RSS sessions could reduce intra-cortical inhibition induced by GABA (gamma-aminobutyric acid) in the motor cortex or could facilitate connections between the primary sensory and primary motor cortical areas, mediated by glutamate [10–11].
However, there is no data about implementing RSS in the acute/early subacute phase of stroke, even though this seems to be the most critical period for neuroplasticity and overall recovery [12]. Therefore, the study developed by Chatterjee et al. [13] aimed to evaluate the feasibility and sustainability of implementing repetitive sensory stimulation programs in the first 2 weeks following a stroke [13].
Study design and participants
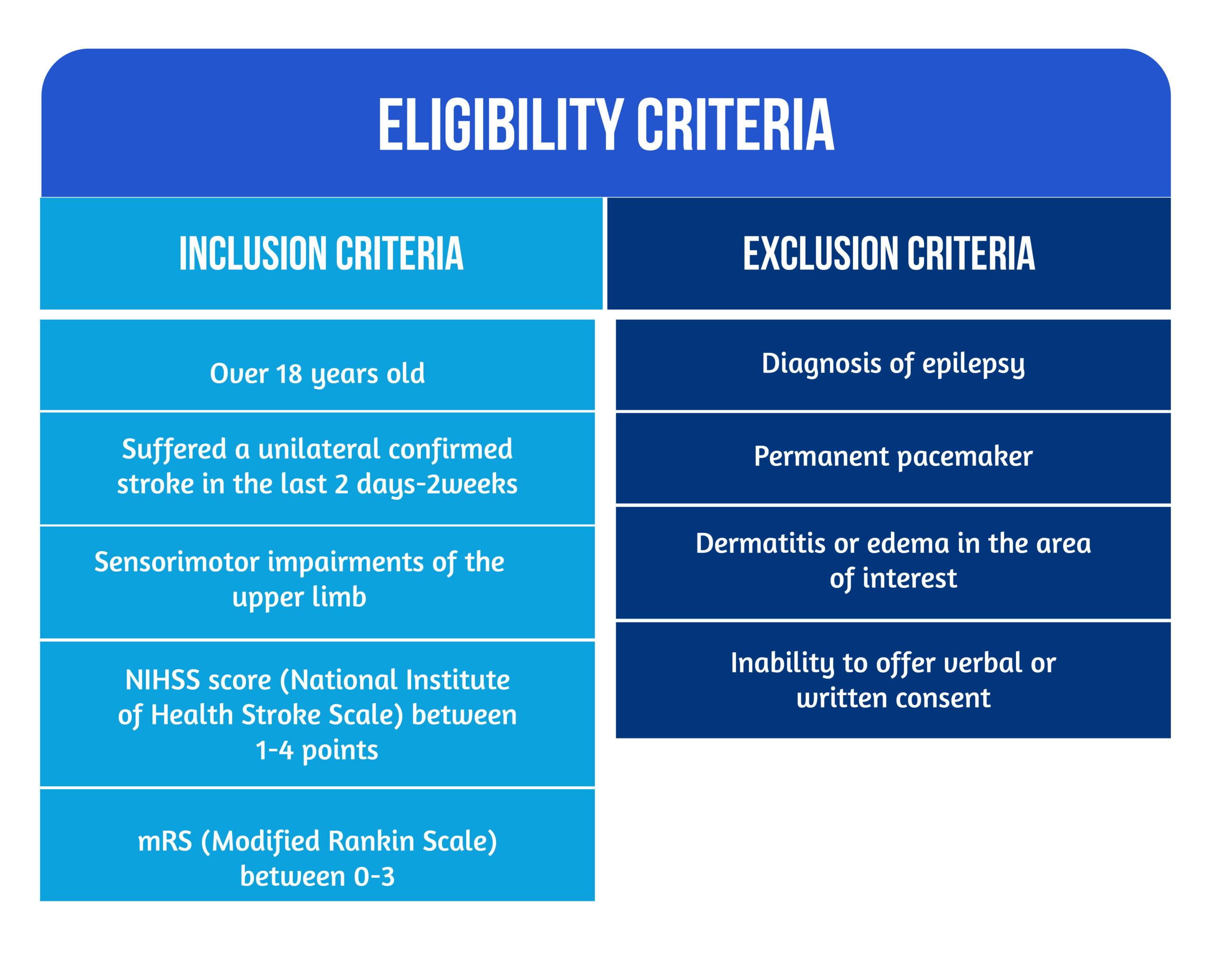
The study conducted by Chatterjee et al. [13] was a multicenter, single-blind, randomized controlled trial in which 40 patients were recruited who met the eligibility criteria [13] (Figure 1) were recruited in stroke units between January and November 2017.
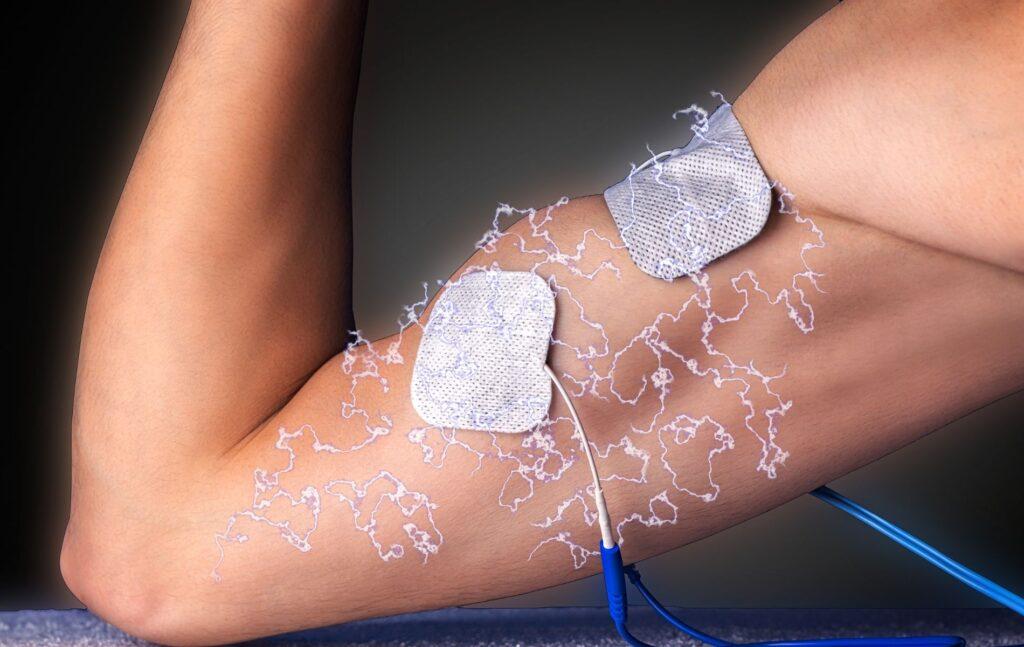
After assessing the inclusion and exclusion criteria, patients were randomized into two groups (experimental- RSS and control- UC groups). An appropriate-sized glove and stimulator box were provided for the repetitive sensory stimulation in the experimental group (23 patients) (Figure 2.). Supra-sensory pulses were used with the help of electrodes placed on the glove’s distal and proximal phalanges. Therefore, stimulation was delivered on the entire area of interest with an intensity of 1 to 20 mA and at a frequency of 20 Hz. The intensity was adapted for each patient and increased to their tolerance level. On the other hand, the UC group (17 patients) received classical neurological rehabilitation with physiotherapists and occupational therapists.
Besides demographic data and the results of the questionnaire developed for the evaluation of patient acceptability of the study, the primary outcome tool was Action Research Arm Test (ARAT). For this test, the scores could indicate
- no upper limb capacity (0-10)
- poor capacity (11-21)
- limited capacity (22-42)
- notable capacity (43-52)
- total capacity (55-57) [14, 15].
Another parameter of interest was the Fugl-Meyer Assessment of the upper extremity outcome tool (FMA-UE), which is highly recommended as an observational measure for upper limb deficits [16]. For a better evaluation of dexterity, the time needed to complete the “nine-hole peg test“ (NHPT) was also used [13]. These tools were reevaluated 2 weeks after completing the sessions and at 3 months follow-ups.
Upper limb repetitive sensory stimulation showed acceptable feasibility in the early phase of the stroke
Of all screened patients, some had mild upper limb deficits so that the intervention would show no benefit; others had pre-existing severe dysfunctions or were unable to give informed consent. The main challenge was to find eligible patients, from whom 77% were recruited.
Finally, 40 patients were included in the 1.5-year study. Rehabilitation treatment included 18 hours per patient in each group. It is possible that the intervention group received physio- and occupational therapy for more extended periods (median time was 21,7 hours in the RSS group versus 18 hours in the UC group). Nevertheless, both groups had the same amount of upper-limb-specific physiotherapy (a median of 3.5 hours). The maximal time per session for sensory stimulation was 45 minutes, and patients underwent 14 sessions.
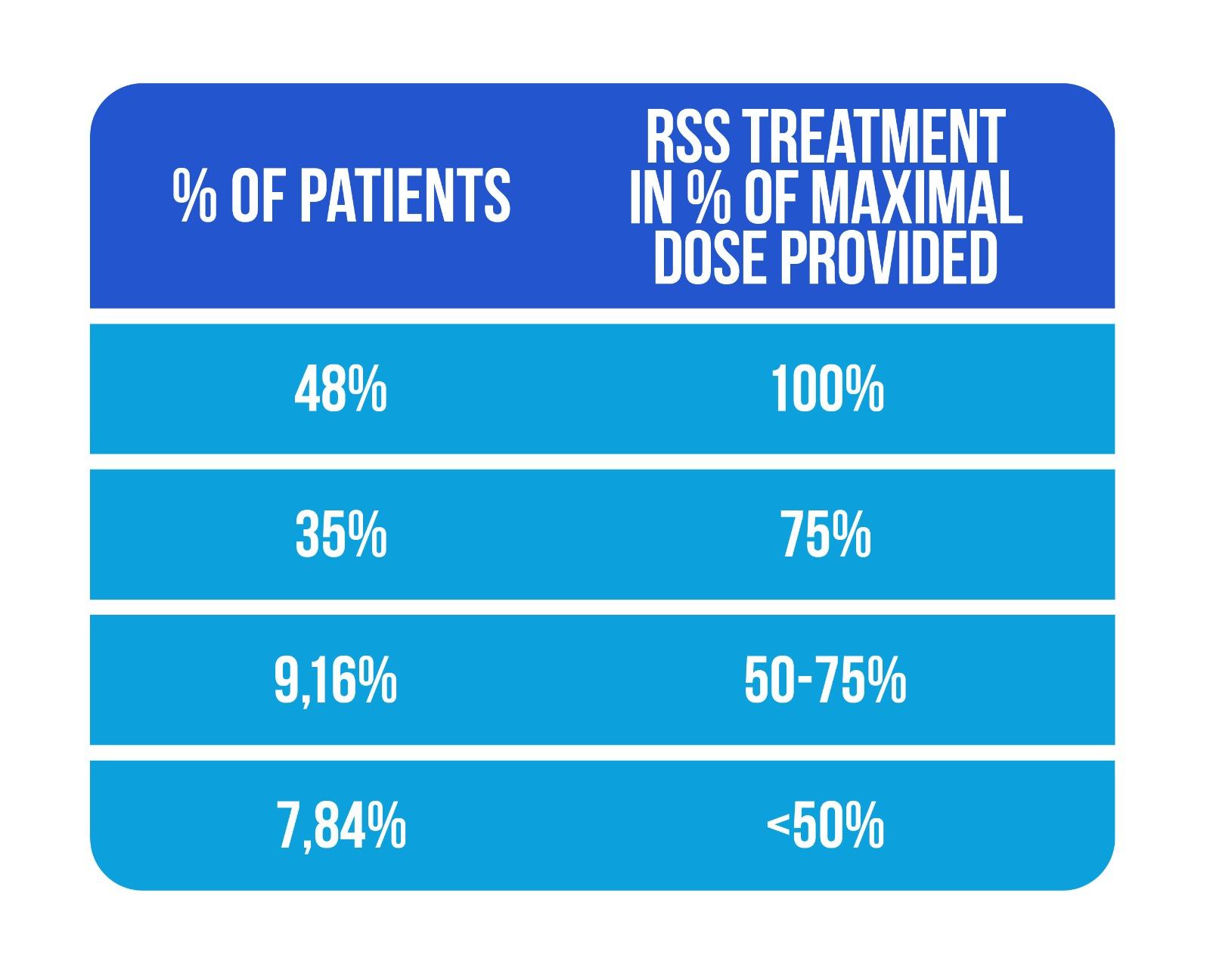
The authors highlighted the good adherence to sensory stimulation treatment in their study, with 83% of patients completing more than 75% of the entire sensory stimulation dose, as shown in Figure 3 (Adapted from Chatterjee et al., [13])
All patients included in both groups returned at 3 months for follow-up analysis, showing good feasibility for this study design.
Upper limb repetitive sensory stimulation is acceptable by early stroke patients
Adverse events of sensory stimulation were rare and minor, with patients reporting shoulder discomfort, hand pain, and dry skin. There were no dropouts from the study. Furthermore, the acceptability of the glove was assessed via questionnaires filled in by almost 50% of the participants. Most patients reported that the glove was easy to use after training, and some reported additional benefits.
Some variables pertaining to the acceptability of the sensory stimulation gloves are represented in Table 1 below.
| Number of patients | Patient’s answers about the acceptability of sensory stimulation glove |
| 2 | Easy to use |
| 6 | Easier only after practice |
| 3 | No effect |
| 3 | Improved hand motility |
| 5 | No negative effects |
| 4 | The glove was tight or slightly painful |
| 5 | Will recommend the glove to other people with a stroke |
| 3 | Will not recommend/will recommend only if it will show benefit |
Table 1. Patient perception
Upper limb sensory stimulation added to conventional rehabilitation programs is efficient in improving hand motor function
In this study conducted by Chatterjee [13], changes in upper limb and hand motor function were measured at the end of the rehabilitation treatment (at 2 weeks) and 3 months follow-up [13]. The scales used demonstrated validity and reliability when applied to stroke rehabilitation [16]. The benefits of the intervention were compared to published values of minimal clinically important differences (MCID) in acute and chronic stroke [14, 15, 17–19].
After rehabilitation, ARAT scores improved in 80% of patients from the RSS group in contrast to 47% from the conventional care group at 2 weeks. At 3 months, 70% of all patients had persistent improvements. The use of the glove for sensory stimulation for 14 sessions makes the patient three times more likely to improve at the end of treatment and 1,5 times more likely to achieve a good motor score at follow-up. Also, patients in the RSS group exceeded the minimal clinically important differences (MCID) in ARAT score, FMA-UE score, and NHPT by more points than patients in the usual care group at 2 weeks and 3 months evaluations, respectively, demonstrating that the use of sensory stimulation is efficient in improving hand motor function when implemented early after stroke [13].
The changes in all outcome tools were significant at 2 weeks but seemed to attenuate at the 3 months evaluation. Thus, this could be an argument for a more extended rehabilitation intervention in the acute phase of stroke.
The authors suggested that the data needs validation in a larger trial of RSS, which will include a more significant number of participants to obtain effectiveness and power to detect positive clinical outcomes of sensory stimulation [13].
Limitations of the study
The study conducted by Chatterjee [13] assessing the feasibility and efficacy of RSS on the affected hand after stroke has some limitations. The duration of intervention was longer in the RSS groups, which could influence the results. Other studies have shown that more intense training sessions will lead to greater improvements in motor scores [20]. The authors pinpointed the necessity of a sham stimulation treatment in the usual care group for future studies [13].
Another limitation was group heterogeneity at inclusion. The RSS group started the intervention 2 days earlier, patients` status before the actual stroke was slightly better on the modified Rankin Scale, but they seemed to have a more severe arm function at baseline. To ensure group equality, the authors [13] suggested the inclusion in future trials of the SAFE score and PREP2 algorithm [21–22].
Why use repetitive sensory stimulation in the rehabilitation of the upper limb?
The researchers designed this single-blinded and randomized study to assess the feasibility and acceptability of a larger study in stroke rehabilitation [13]. The intervention used was repetitive sensory stimulation applied on a glove on the affected hand of stroke patients, administered over usual rehabilitation interventions, and was the first study to include patients in the early phase after stroke. The study design is feasible, but the inclusion of patients from many stroke centers would be necessary for future research to obtain the optimal amount of data needed to prove effectiveness. The patients tolerated the sensory stimulation well, adhering to the intervention. Patient satisfaction after RSS was also noticeable. Nevertheless, more homogenous randomization into groups using more sensitive scales for upper limb function should improve data accuracy [13].
Conclusions
The RSS intervention was beneficial and improved arm function measured by any study scales. Patients who used the glove for sensory stimulation were 3 times more likely to achieve a better motor outcome at 2 weeks and 1,5 times at 3 months, but the differences were not statistically significant. A strong point of this study was that improvements in motor scales were compared with the spontaneous evolution of motor deficits after stroke, called the minimal clinical important difference (MCID). Patients with RSS therapy had scores that exceeded MCID with more points than patients with conventional therapy alone, suggesting the efficacy of the intervention [13].
Further studies on large groups of patients with longer interventions are required to assess the efficacy of repetitive sensory stimulation and its place in the rehabilitation of hand function in stroke patients.
References
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, et al. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383(9913):245–254 doi: 10.1016/s0140-6736(13)61953-4
- Chen CM, Tsai CC, Chung CY, Chen CL, Wu KP, Chen HC. Potential predictors for health-related quality of life in stroke patients undergoing inpatient rehabilitation. Health Qual Life Outcomes. 2015;13:118. https://doi.org/10.1186/s12955-015-0314-5
- Broeks JG, Lankhorst GJ, Rumping K, Prevo AJ. The long-term outcome of arm function after stroke: results of a follow-up study. Disabil Rehabil. 1999;21(8):357–364. DOI: 10.1080/096382899297459
- French B, Thomas LH, Coupe J, McMahon NE et al. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst Rev. 2016;11:CD006073. https://doi.org/10.1002/14651858.
- Cortes JC, Goldsmith J, Harran MD, Xu J, et al. A short and distinct time window for recovery of arm motor control early afterstroke revealed with a global measure of trajectory kinematics. Neurorehabil Neural Repair. 2017;31(6):552–560. https://doi.org/10.1177/
- Party ISW. Sentinel Stroke National Audit Programme; 2017.
- Hayward KS, Brauer SG. Dose of arm activity training during acute and subacute rehabilitation post stroke: a systematic review of the literature. Clin Rehabil. 2015;29(12):1234–1243. https://doi.org/10.1177/0269215514565395.
- Beste C, Dinse HR. Learning without training. Curr Biol. 2013;23(11):R489–R499. https://doi.org/10.1016/j.cub.2013.04.044.
- Kattenstroth JC, Kalisch T, Sczesny-Kaiser M, Greulich W et al. Daily repetitive sensory stimulation of the paretic hand for the treatment of sensorimotor deficits in patients with subacute stroke: RESET, a randomized, sham-controlled trial. BMC Neurology. 2018;18:1–13. https://doi.org/10.1186/s12883-017-1006-z.
- Celnik P, Hummel F, Harris-Love M, Wolk R, Cohen LG. Somatosensory stimulation enhances the effects of training functional hand tasks in patients with chronic stroke. Arch Phys Med Rehabil. 2007;88(11):1369-76. doi: 10.1016/j.apmr.2007.08.001.
- Veldman MP, Maffiuletti NA, Hallett M, Zijdewind I, Hortobagyi T. Direct and crossed effects of somatosensory stimulation on neuronal excitability and motor performance in humans. Neurosci Biobehav Rev. 2014;47:22–35. doi.org/10.1016/j.neubiorev.2014.07.013
- Bernhardt J, Hayward KS, Kwakkel G, Ward NS, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: The Stroke Recovery and Rehabilitation Roundtable Taskforce. Neurorehabil Neural Repair. 2017;31(9):793–799. https://doi.org/10.1177/1545968317732668.
- Chatterjee, K., Stockley, R.C., Lane, S. et al.PULSE-I – Is rePetitive Upper Limb SEnsory stimulation early after stroke feasible and acceptable? A stratified single-blinded randomised controlled feasibility study. Trials20, 388 (2019). https://doi.org/10.1186/s13063-019-3428-y
- Van der Lee JH, De Groot V, Beckerman H, Wagenaar RC et al. The intra- and interrater reliability of the action research arm test: a practical test of upper extremity function in patients with stroke. Arch Phys Med Rehabil. 2001;82(1):14–19. https://doi.org/10.1053/apmr.2001.18668. = 16
- van der Lee JH, Beckerman H, Lankhorst GJ, Bouter LM. The responsiveness of the Action Research Arm test and the Fugl-Meyer Assessment Scale in chronic stroke patients. J Rehabil Med. 2001;33(3):110–113. DOI: 10.1080/165019701750165916
- Kwakkel G, Lannin NA, Borschmann K, English C et al. Standardized measurement of sensorimotor recovery in stroke trials: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Neurorehabil Neural Repair. 2017;31(9):784–92 https://doi.org/10.1177/1545968317732662.
- Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther. 1985 Jun;39(6):386-91. doi: 10.5014/ajot.39.6.386.
- Lang CE, Edwards DF, Birkenmeier RL, Dromerick AW. Estimating minimal clinically important differences of upper-extremity measures early after stroke. Arch Phys Med Rehabil. 2008;89(9):1693–1700. https://doi.org/10. 1016/j.apmr.2008.02.022
- Page SJ, Levine P, Hade E. Psychometric properties and administration of the wrist/hand subscales of the Fugl-Meyer Assessment in minimally impaired upper extremity hemiparesis in stroke. Arch Phys Med Rehabil. 2012;93(12):2373–6.e5. https://doi.org/10.1016/j.apmr.2012.06.017
- Peurala SH, Pitkanen K, Sivenius J, Tarkka IM. Cutaneous electrical stimulation may enhance sensorimotor recovery in chronic stroke. Clin Rehabil. 2002;16(7):709 –716. https://doi.org/10.1191/0269215502cr543oa
- Stinear CM, Barber PA, Petoe M, Anwar S, Byblow WD. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain. 2012;135(Pt 8): 2527 –2535. https://doi.org/10.1093/brain/aws146
- Stinear CM, Byblow WD, Ackerley SJ, Smith MC et al. PREP2: a biomarker-based algorithm for predicting upper limb function after stroke. Ann Clin Transl Neurol. 2017;4(11):811 –820. https://doi.org/10. 1002/acn3.488










One thought on “The feasibility of repetitive sensory stimulation in the rehabilitation of the upper limb after a stroke (the PULSE-I study)”