Neuroplasticity and aging – is there a relation?

Authors: Irina Benedek, Oana Vanta
Keywords: aging, neuroplasticity, deep brain stimulation, neurogenesis, neuroimaging
Neuroplasticity and aging – is there a relation?
Neuroplasticity is a final common direction of neurobiological processes, such as structural, functional, or molecular mechanisms, that generate stability or compensation for changes brought on by aging or illness [1].
To develop neuroplasticity into clinical intervention paradigms, translation between research in animals and humans and crosstalk between neurological and psychiatric pathologies is essential. While most fundamental studies have typically concentrated on “critical periods” of early development and the potential to induce neuroplasticity in adulthood or during another key phase, the aging process has recently received increased attention [1].
Are there any strategies that can induce neuroplasticity?
Neuroplasticity could be promoted by the following interventions:
- behavioral (physical exercise and cognitive activity)
- physiological (caloric restriction)
- pharmacological (modulation of AMPA receptors)
- brain stimulation techniques (transcranial magnetic stimulation [TMS], magnetic seizure therapy [MST], and deep brain stimulation [DBS])
- TMS with pharmacologic therapy efficient treatment of cognitive impairments following a stroke or dementia
- Epigenetic modifications (histone deacetylase inhibitors)
- Estrogen treatment
- Interventions on neuroinflammatory processes, together with the last two treatment options, need further studies to be applicable in neuropsychiatric pathologies
- Environmental and lifestyle variables should be the subject of research and the generation of new treatments [1–4].
How do neurotransmitter interactions modulate neuroplasticity?
In a study conducted by Mora et al., preclinical evidence showed that regional neurotransmitter interactions in functionally related systems (in this example, glutamate regulation of dopamine and GABA) might alter with age, especially under stressful circumstances. This hypothesis has several significant implications:
- First of all, although glutamate is involved in cell death and the preservation of cellular function, it is unclear how glutamate is modulated in aging and neurodegenerative processes in the human brain. To maintain synaptic and dendritic plasticity, several glutamatergic transporters and receptors are essential [5].
- Second, the primary target and functionally connected neurotransmitters are involved in psychotropic drugs’ mechanism of action [1].
- Levodopa therapy in Parkinson’s disease causes serotonin neurons in the raphe to generate dopamine.
- The impact of selective serotonin reuptake inhibitors on post-stroke motor performance is another example; these effects may also impact the dopaminergic and glutamatergic systems [1].
By having a more substantial impact on a neurotransmitter downstream from the primary effect, neuroplasticity associated with different therapies (such as TMS, MST, and DBS) may be able to restore the balance between functionally connected systems or trigger a therapeutic response [1].
Can neuroplasticity be measured?
In preclinical studies, increased production of synaptic proteins and trophic factors (such as brain-derived neurotrophic factor – BDNF) that promote neurogenesis or remodeling of the spine and dendritic architecture is frequently seen as signs of neuroplasticity at the molecular level [5].
Neuroplasticity is also thought to represent changes in structural and functional brain imaging. There are many instances of neuroimaging data interpreted as proof of neuroplasticity in the literature (Figure 1) [1].

To address basic concerns of functional relevance, the neuroimaging data must be interpreted in the context of behavioral and clinical outcomes, specifically, if functional gains are related to changes in cerebral blood flow/glucose metabolism, white matter functional connectivity, and brain volume (Figure 2).
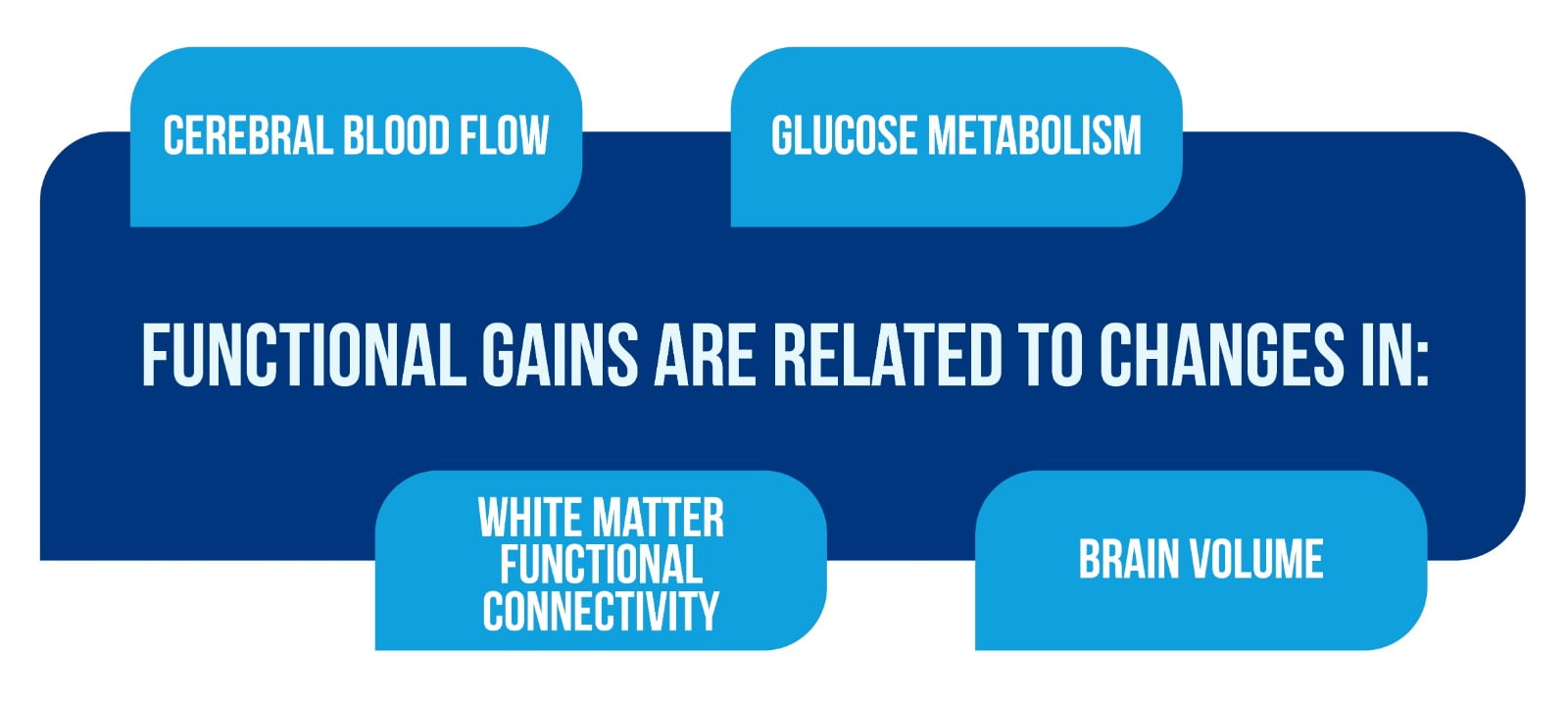
To interpret neuroimaging results by underlying neurobiological mechanisms, particularly the observed changes as proof of neuroplasticity, combined neuroimaging and histopathologic or neuropathological evaluations in animal models and the human brain, respectively, are crucial [1].
Is the neuroplasticity process important in the development of new treatments?
Their impact on neuroplasticity could measure the effect of innovative therapies. There are various concepts in the literature where preclinical research has focused on innovative therapies that have been demonstrated to be helpful in patients. These studies were based on clinical and/or imaging evidence that found proof of neuroplasticity or neurogenesis as a mechanism of action. Translational research on selective serotonin reuptake inhibitors is the finest illustration [5].
More recently, preclinical studies have demonstrated that a single dose of ketamine is associated with increased levels of synaptic proteins and increased number and function of axon-spinous synapses of pyramidal neurons in a rat’s prefrontal cortex.
- These studies were motivated by the evidence for a rapid antidepressant action of the N-Methyl-D-aspartate (NMDA) receptor antagonist, ketamine, in treatment-resistant depressed patients [6].
- Additional research into the immediate antidepressant effects of ketamine would significantly advance the understanding of neuroplasticity and antidepressant response in the human brain, particularly of the NMDA receptor [1].
What are the effects of Deep brain stimulation (DBS) on neuroplasticity?
Examples of possible DBS effects on neuroplasticity and connections with therapeutic advantages may be found throughout the literature.
- Bewernick and Schlaepler examined the preclinical findings addressing the effects of DBS on hippocampus neurogenesis and the substantial evidence supporting the antidepressant benefits of DBS in treatment-resistant depression.
- Adaptive alterations in cerebral blood flow in brain networks linked to depression have been observed in neuroimaging studies conducted throughout DBS, which may reflect neuroplasticity-related underpinning processes.
- Recent studies in Alzheimer’s disease (AD) have demonstrated that in contrast to the decreased metabolism and decreased functional connectivity seen throughout AD, 1 year of continuous DBS (anterior to the columns of the fornix) increased cortical glucose metabolism and functional connectivity.
- Neurogenesis and the production of neurotrophic factors, such as BDNF, were shown in preclinical trials of DBS of Papez’s circuit, which may explain the metabolic changes observed [1, 7].
Neuroplasticity and aging process – what is the connection?
Except for memory training programs, which are quickly expanding, there is evidence for neuroplasticity in aging animals and human brains, but there are few clinical studies of neuroplasticity-promoting treatments [1].
Neuroimaging studies and peripheral biomarker assessments during treatment in both preclinical models and humans are important opportunities to obtain information.
Some significant research opportunities include clinical trials of interventions for the following subjects, presented in Figure 3 [1].

The study of neurodegenerative diseases has provided insight into maximizing the impact of such therapies, which might also be applied to aging. Regarding the time of the intervention, many lines of research suggest that the effects of a specific treatment may be at peak immediately after brain damage or in the early stages of a disease process:
- The greatest effect of rehabilitation was shown in stroke cases immediately after damage when attempts to rehabilitate animals could take advantage of the brief window of neuroplasticity that has been demonstrated to occur.
- Research on AD and cognitive aging increasingly concentrates on prevention in “asymptomatic” people at risk due to genetic or neuroimaging indications of a neurodegenerative process, family history, or both. Early intervention in these people may prevent the loss of synapses and dendritic spines that result from the buildup of β-amyloid. [1].
Neuroplasticity and aging | What is the conclusion?
A range of therapies that show neuroplasticity in preclinical models has been studied.
Further lessons and avenues for development include:
- Establishing whether structural and functional neuroimaging findings in humans genuinely represent neurogenesis. For this, translational studies of human models that combine neuroimaging with histological or neuropathological analysis are required.
- Understanding the nature of neuroplasticity in humans. This process will progress thanks to multi-modality neuroimaging investigations that correlate the structural and functional change to molecular and neurochemical processes.
- Exploring exceptional opportunities to use neuroimaging methods to develop treatment algorithms based on a combination of interventions. This could include the effects of behavioral and environmental manipulations and brain stimulation.
- Identifying “at-risk” individuals for prevention trials.
For more information on neurorehabilitation, visit:
- What is the impact of early life on brain and cognitive reserve?
- Does exercise induce neuroplasticity?
- Efficacy of placebo in managing pain for neurological disorders
We kindly invite you to browse our Interview category: https://efnr.org/category/interviews/. You will find informative discussions with renowned specialists in the field of neurorehabilitation.
References
- Smith GS. Aging and neuroplasticity. Dialogues Clin Neurosci. 2013;15(1):3-5. doi: 10.31887/DCNS.2013.15.1/gsmith.
- Luber B, McClintock S, Lisanby SH. Applications of transcranial magnetic stimulation and magnetic seizure therapy in the study and treatment of disorders related to cerebral aging. Dialogues Clin Neurosci. 2013;15(1):87–98. doi: 10.31887/DCNS.2013.15.1/bluber
- Day JJ, Sweatt JD. Epigenetic treatments for cognitive impairments. Neuropsychopharmacology. 2012;37:247–260. doi: 10.1038/npp.2011.85
- Erickson K, Gildengers A, Butters M. Physical activity and brain plasticity in late adulthood. Dialogues Clin Neurosci. 2013;15(1):99–108. doi: 10.31887/DCNS.2013.15.1/kerickson
- Licznerski P, Duman RS. Remodeling of axo-spinous synapses in the pathophysiology and treatment of depression. Neuroscience. 2012. DOI: 10.1016/j.neuroscience.2012.09.057
- Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. DOI: 10.1126/science.1190287
- Bewernick B., Schlaepfer T. Chronic depression as a model disease for cerebral aging. Dialogues Clin Neurosci. 2013;15(1):77–85. doi: 10.31887/DCNS.2013.15.1/bbewernick