Constraint-Induced Movement Therapy improves short-term motor function of early stroke patients

Authors: Ioana Stanescu, Oana Vanta
Keywords: stroke rehabilitation, upper limb rehabilitation, arm-hand function recovery
Rehabilitation treatment – efficient in the early phase of stroke | Constraint-induced movement therapy
Rehabilitation is an essential component of stroke therapy, with important benefits if initiated in the acute phase. In the first 3 weeks after a stroke, rehabilitation is initiated in the stroke unit and continued during hospitalization. In the subacute stage, the treatment continues in inpatient rehabilitation hospitals, community-based centers, or skilled nursing facilities, depending on stroke severity and predicted outcome [1].
In the acute stroke phase, around 80% of patients have upper extremity impairment, which persists in the sub-acute phase in almost half of the patients [2]. Disability is a dramatic consequence in 50% of stroke survivors, while one-third lose their autonomy and become dependent on others for daily living [3]. After a stroke, the perspective of regaining hand function is essential for patients. Upper limb motor deficit is strongly correlated with disability and is also a negative predictor of the quality of life after stroke [4].
In the first weeks after stroke, there is an enhanced neuroplasticity process, with an increased ability for learning new activities, as evidence indicated. Neuroplasticity refers to the ability of the nervous system to change and adapt after an injury; it has been proven that this process can be enhanced by active, repetitive, and task-specific movements of the upper limb [5].
For more information about neurorehabilitation, visit:
- Post-stroke upper limb deficits – The EXPLICIT Trial
- SENSe: Assessing neurorehabilitation’s impact on the somatosensory function in stroke patients
- Rehabilitation of non-fluent aphasia patients
Constraint-induced movement therapy (CIMT) – a rehabilitation method for stroke patients with upper limb motor deficits
Constraint-induced movement therapy (CIMT) belongs to a family of rehabilitation techniques dedicated to patients with unilateral upper limb motor deficits. This method promotes excessive use of the hemiparetic upper limb by restraining the movements of the unaffected arm. The constraint of movements in the less affected (unaffected) hand uses different methods: wearing a padded mitt, a glove, or a splint, associated with constant reminders provided to the patient. The patient is required to use only the paretic hand almost 90% of the time awake and while performing specific training for many hours per day for 2 or 3 consecutive weeks [6–7].
CIMT is considered one of the most effective methods of physical therapy in improving upper limb motor deficits. The efficacy is driven by repetitions and task-specific motor learning [8].
The components of CIMT are [8]:
- Restriction of the use of the non-affected arm by immobilization with a padded mitt for almost all day long, called forced-use therapy
- Intensive practice with the paretic limb of repetitive and task-oriented exercises (for up to 6 hours/day for 2 weeks)
- Development of behavioral strategies that increase compliance and adherence to training (transfer of the practiced tasks in patient`s daily life, treatment contract, motor activity log, home diary).
The tasks practiced during CIMT and the restricted use of the non-paretic hand are shown in Figure 1.
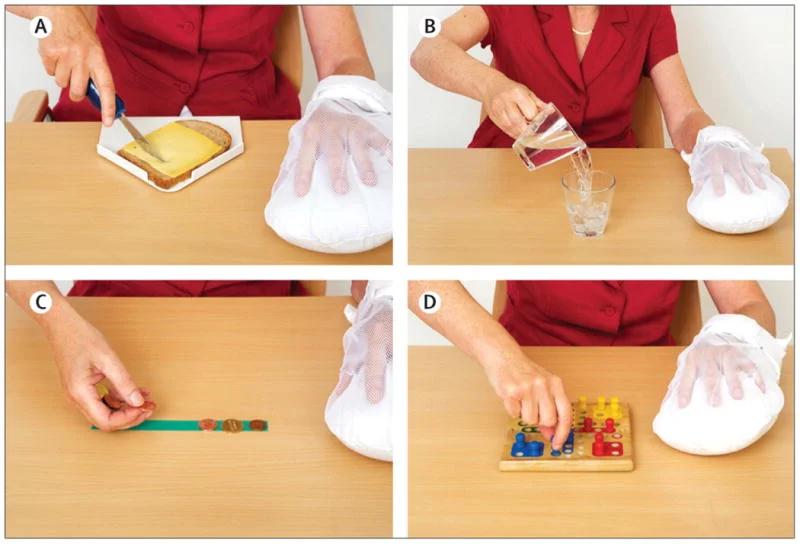
Figure 1: Constraint-Induced Movement Therapy: task-oriented practice with the affected hand (A), (B), specific exercises (C), (D). The non-affected hand is constrained with a mitt – From Kwakkel et al. [8].
CIMT improves motor function and arm-hand activities in chronic stroke patients
Many CIMT protocols have been developed for chronic stroke patients (patients included at >3 months after stroke onset). Mark and Taub developed in 2004 the original protocol for patients with chronic stroke, with a constraining duration of the non-affected arm for 90% of the time awake and with high intensity of the therapy (6 hours/day, for 10 days) [9].
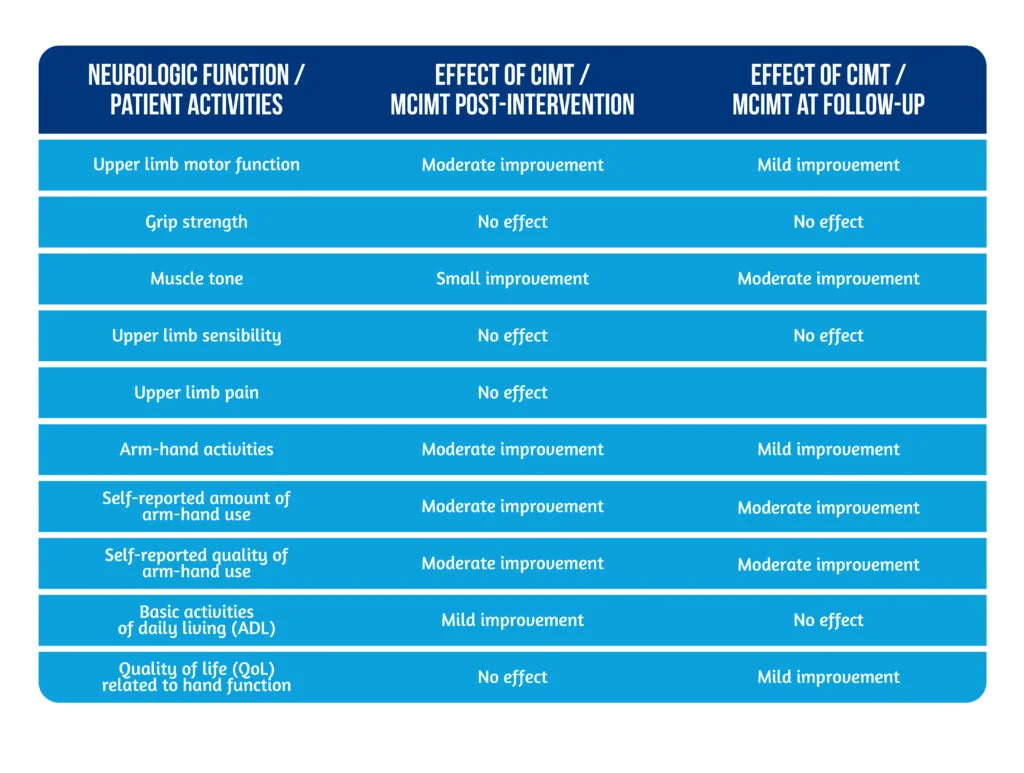
Many other modified versions of CIMT (mCIMT) were investigated in randomized controlled trials. The results of original and modified versions were similar: small to medium improvements in the motor function of the paretic arm, arm-hand activities, and increased self-reported amount and quality of affected arm and hand use during daily activities. The effects were reported at the end of the intervention and were maintained at long-term follow-up (after 4 months) [8–9].
The benefits of CIMT and mCIMT on different neurologic functions, patient activities, and participation in daily life are shown in Table 1 (adapted from Kwakkel [8]).
The efficiency of CIMT in acute stroke rehabilitation
Only a few trials investigating CIMT in acute stroke rehabilitation have been conducted, with limited evidence and variable results. The duration and intensity of CIMT are under debate, and extensive studies are needed.
A group of Norwegian authors conducted a multicenter, single-blinded, randomized, controlled trial for assessing CIMT results in the early stage of stroke patients – The Norwegian CI therapy multisite trial (NORCIMT) [10]. The authors included patients with a recent stroke (onset 5 to 26 days before inclusion) with a partial motor deficit in the arm and hand (the patient was able to extend 2 fingers of the hand in pronation on a horizontal plane or to extend the flexed wrist by 10 degrees) and without dementia or prior disability.
The intervention group included 24 patients who received CIMT 3 hours per day for 10 consecutive working days (2 weeks). A constraining mitt was worn on the paretic hand 90% of the time during which the participants were awake. Also, these patients received standard rehabilitation treatment consisting of physical therapy and occupational therapy sessions, according to national guidelines. The control group of 23 patients received only standard rehabilitation treatment with the same intensity and for the same amount of time. Assessments were made at inclusion, at the end of the intervention – 2 weeks and at 6 months.
The primary outcome was the change in the arm motor function after the treatment, measured by the Wolf Motor Function test (WMFT). The secondary outcomes of the NORCIMT trial were to evaluate the effect of CIMT on arm dexterity (measured by the Nine-Hole Peg test NHPT), arm use in daily activities (assessed by an accelerometer worn for 24 hours on each arm), and global health status post-stroke (evaluated by the Stroke Impact Scale questionnaire) [10].
The safety and feasibility of CIMT in acute stroke patients
The authors [10] pinpoint that the majority of patients (20 from 24) completed all treatment sessions. Two patients withdrew after initiating treatment, and two were lost to a 6-months follow-up. The adverse events were mild and included 1 case of shoulder pain and 1 case of shoulder capsulitis.
There was a concern about safety regarding the possible balance problems in patients with a constraint-unaffected upper limb, but no events were reported. Thus, CIMT is safe, well tolerated, and feasible in patients in the early stroke phase [10].
CIMT might enhance motor recovery in the acute stroke phase
This study by Thrane and collab. [10] shows significant improvement in the arm motor function in patients treated with CIMT at the end of the intervention, as demonstrated by the WMFT score. The patients from the CIMT group obtained better times in completing motor tasks and improved functional ability scores. No differences in arm or grip strength were detected between the 2 groups of patients [10]. A moderate improvement in the dexterity of the affected hand was detected after CIMT treatment (NHP test). The beneficial effect of CIMT on arm motor function and hand dexterity was not sustained at 4 months follow-up; both groups of patients showed similar improvement in all assessed parameters.
The authors hypothesized that the positive effect of CIMT in early stroke could be related to the facilitation of the learning process and of neurological repair mechanisms in acute stroke patients. This study provides important new evidence on CIMT application in early post-stroke rehabilitation [10].
Conclusion
Constraint-Induced Movement Therapy (CIMT) is a rehabilitation intervention that aims to increase the use of the paretic limb by restraining the non-affected limb movements for almost 90% of the active time. The affected limb is trained to perform highly repetitive and task-specific movements. Behavioral strategies included in the CIMT improve patient compliance (by contracts, logs, diaries) and provide support for the transfer of the acquired motor skills in daily life activities [6-8].
CIMT is considered one of the most effective methods of physical therapy in improving upper limb motor deficits. Its effects in improving motor function and muscle tone in the arm and hand, arm-hand activities, and the amount and quality of arm-hand use in daily life, have robust evidence in chronic stroke. The effects are manifested at the end of the treatment period and are persistent for 4-6 months [8–9].
The use of CIMT in the first 28 days after the stroke improves early motor function and accelerates the recovery process. A recent trial showed that CIMT is safe and well tolerated in early stroke patients. CIMT induces short-term improvements in arm motor function and hand dexterity, but the effects are not persistent. More trials of CIMT in early stroke are needed for optimal results, adjusting the duration or intensity of the intervention [10].
Bibliography
- Winstein CJ, Stein J, Arena R, Bates B, et al; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2016;47(6):e98-e169. doi: 10.1161/STR.0000000000000098.
- Rafsten L, Meirelles C, Danielsson A, Sunnerhagen KS. Impaired Motor Function in the Affected Arm Predicts Impaired Postural Balance After Stroke: A Cross-Sectional Study. Front Neurol. 2019;10:912. doi: 10.3389/fneur.2019.00912
- Markus HS. Reducing disability after stroke. International Journal of Stroke. 2022;17(3):249-250. doi:10.1177/17474930221080904
- Pomeroy V, Aglioti SM, Mark VW, McFarland D et al. Neurological principles and rehabilitation of action disorders: rehabilitation interventions. Neurorehabil Neural Repair. 2011;25(5 Suppl):33S-43S. DOI: 10.1177/1545968311410942
- Zhang C, Li-Tsang CWP, Au RKC. Robotic approaches for the rehabilitation of upper limb recovery after stroke. Int J Rehabil Res.2017;40:19-28 DOI: 10.1097/MRR.0000000000000204
- Taub E, Uswatte G, Pidikiti R. Constraint-Induced Movement Therapy: a new family of techniques with broad application to physical rehabilitation–a clinical review. J Rehabil Res Dev. 1999;36(3):237-51. Available at: https://pubmed.ncbi.nlm.nih.gov/10659807/
- Morris DM, Taub E, Mark VW. Constraint-induced movement therapy: characterizing the intervention protocol. Eura Medicophys. 2006;42(3):257-68. Available at: https://pubmed.ncbi.nlm.nih.gov/17039224/
- Kwakkel G, Veerbeek JM, van Wegen EE, Wolf SL. Constraint-induced movement therapy after stroke. Lancet Neurol. 2015;14(2):224-34. doi: 10.1016/S1474-4422(14)70160-7.
- Mark VW, Taub E. Constraint-induced movement therapy for chronic stroke hemiparesis and other disabilities. Restor Neurol Neurosci. 2004;22:317-336. Available at: https://pubmed.ncbi.nlm.nih.gov/15502259/
- Thrane G, Askim T, Stock R, Indredavik B, Gjone R, Erichsen A, Anke A. Efficacy of Constraint-Induced Movement Therapy in Early Stroke Rehabilitation: A Randomized Controlled Multisite Trial. Neurorehabil Neural Repair. 2015;29(6):517-25. doi: 10.1177/1545968314558599.