Neurorehabilitation therapies after stroke – what therapies are available?
Authors: Livia Popa, Oana Vanța
Keywords: post-stroke neurorehabilitation, non-invasive therapies, treadmill training, constraint-induced therapy, prism adaptation training, virtual reality in stroke rehabilitation
Focus keyword: neurorehabilitation therapies
Introduction – neurorehabilitation therapies after stroke
The human brain is arguably one of the greatest wonders in the Universe. It is incredibly powerful and infinitely complex yet fragile. After a stroke, simple actions can become intimidating challenges when blood supply is basically cut off to a certain part of the brain. Sensation, balance, movement, cognition, or speech may be affected in various degrees and combinations. Independence may be temporarily or permanently lost.
Fortunately, the conventional wisdom that brain or nerve damage is permanent has been replaced by a new paradigm. Fascinating research and innovative therapies are developed based on the brain’s proven neuroplasticity. When adequately trained or stimulated, the brain can reorganize and reestablish connections with the body and within itself.
This factsheet presents a complex approach to neurorehabilitation therapies, including some general principles of successful neurorehabilitation and five examples of non-invasive therapies for people seeking to recover after a stroke. as presented in Figure 1 below:

For more information on neurorehabilitation therapies, visit:
- Efficacy of virtual reality augmented robot-assisted gait training in chronic stroke – results from a randomized controlled single-blind trial.
- Shedding light on Speech-Language Therapy: How stroke survivors can benefit from intensive rehabilitation training for chronic aphasia
- Robot-assisted neurocognitive rehabilitation of the hand
What makes neurorehabilitation after a stroke effective?
According to research, the principles of brain plasticity and motor learning behind effective neurorehabilitation therapies or programs are:
- Task-specific and goal-oriented practice, for example, the meaningful execution of movements that are part of basic daily activities
- Variable practice and increasing difficulty, which can also prevent boredom
- Multisensory stimulation, for example tasks that engage the patient’s hearing and eyesight
- Explicit and implicit feedback, such as verbal feedback given by therapists as well as the immediate results of the patient performing a certain task
- Social interaction, including among patients, such as in pairs of patients performing therapeutic exercises together [1].
Motor neurorehabilitation therapies or programs can consist of:
- a treadmill for walking training
- constraint-induced language therapy for speech recovery
- prism adaptation training for spatial neglect.
Is it safe to use a treadmill to practice walking after a stroke?
For patients left with a walking disability after a stroke, treadmill training can be safe and beneficial if done in a controlled setting. This approach is labor-intensive and focuses on functional movement. A treadmill training program for post-stroke patients often begins with slow walking motions guided by one or more technicians, while the patient’s weight is reduced with the help of a weight-supporting harness [2].
According to more than 50 trials on more than 3,000 patients (with an average age of 60 years), treadmill training helps people who had a stroke improve their ability to walk and their walking speed. Notably, treadmill training is effective for patients who start the therapy process with some ability to walk unaided. For this population, the risk of falling off the treadmill is no greater than in the case of healthy individuals who used treadmills [3].
What is constraint-induced therapy?
After a stroke, it is not unusual for recovering patients to struggle to use their arms in the same way as before. Typically, one arm is fine, while the other becomes hard to move or control.
Basic and instrumental daily activities can prove technically challenging and emotionally frustrating:
- activities involving personal hygiene
- dressing
- shopping
- cooking
- eating
- housekeeping
- using the toilet.
In such situations, patients tend to compensate and use their “good” arm to do what the other can no longer do. As tempting as this can be, it is hardly a solution to the actual problem.
Constraint-induced therapy is beneficial in rehabilitation programs for motor and speech recovery (Figure 2).
To retrain motor function and control of an upper limb, constraint-induced movement therapy (CIMT) aims to restore the patient’s ability with their affected arm by limiting their use of the healthy one, such as by wearing a glove over the healthy hand. In this way, the person uses the affected arm according to an individually tailored exercise plan and during waking hours to perform basic everyday activities. One condition is, however, that the patient still has some preserved movement to begin with [2].

Does constraint-induced therapy actually work?
According to a thorough review of the research, CIMT is practical but still needs more evidence. Studies have shown that CIMT can help patients improve arm movement better than conventional physiotherapy or no treatment. However, the scientific data is still inconclusive regarding the long-term benefits of CIMT in daily activities [4].
An interesting fact about constraint-induced therapy is that it can also be applied to the recovery of language and speech function in post-stroke patients with aphasia. People struggling to speak often adapt by relying more on hand gestures and other non-verbal behaviors to convey the message. By applying physical barriers that limit non-verbal communication, game-like techniques can be used to stimulate verbal production and/or interaction. This is called constraint-induced language therapy (CILT), or constraint-induced aphasia therapy (CIAT), and studies have shown that it can be just as or even more effective than other intensive methods, mainly if the therapy process features social interaction [2, 5].
How can spatial misperception and neglect be treated?
Spatial errors due to visual dysfunction and/or motor control issues are quite common after a stroke. Spatial miscalculations and inaccurate body movements like asymmetric reporting/response are generically called spatial neglect [2].
Most spatial neglect therapies consist of visually informed motor tasks. Prism adaptation training (PAT) is a method delivered in short, intensive sessions in which the patient performs specific manual actions while wearing distorting prism lenses (see Figure 3). At first, patients miscalculate in the direction of the optical distortion, and then, through repetition, they become increasingly precise. When they take the special glasses off, they initially make mistakes in the opposite direction but adapt more quickly to their surroundings [2].

What is transcranial direct current stimulation? Is it the same as transcranial magnetic stimulation?
Transcranial direct current stimulation (tDCS) is a non-invasive way of running an electrical current through the patient’s brain in a careful and controlled way. The excitation of the cortex can be both safe and helpful in a wide range of neurological and psychiatric conditions, including some that may be caused by a stroke event, such as:
- motor dysfunction
- aphasia
- spatial neglect.
On the one hand, this method is relatively cheap and easy to implement. On the other, for best results, it needs careful calibration and an experienced operator [2].
Did you know? Transcranial electrical stimulation as a therapeutic practice is about… 200 years old! [2]
Similarly (but not identically), transcranial magnetic stimulation (TMS) is another non-invasive technique where an electrical current is used to generate a magnetic field, which is then sent through the brain. It is mainly used to assess and diagnose neurological conditions and to monitor the patient’s brain during surgery. When the same principles are used to downregulate or upregulate the excitation of the brain’s cortical areas through repetitive stimulation (rTMS), this method was shown to produce therapeutic benefits for patients recovering from stroke or suffering from chronic pain, PTSD, etc. [2]. However, a review of published studies recently highlighted the need for more and better-quality scientific evidence before recommending mainstreaming rTMS in clinical practice [6].
Virtual reality and post-stroke neurorehabilitation?
Virtual reality (VR) experiences are now an industry-standard in video games and entertainment, but VR has many medical applications, too. Computer-based simulations of real-life objects, activities, and events are designed to immerse the patient in intensive, interactive practice with instant feedback that can otherwise be too difficult, boring, expensive, dangerous, or even impossible to set up conventionally in a hospital or home setting. For example, a relevant scenario for walking speed practice is crossing the street, which in VR can be recreated safely as many times as necessary and with varying degrees of difficulty [7].
VR neurorehabilitation applications are being developed all the time now that VR technology has become ubiquitous and affordable. A comprehensive review of more than 70 such VR programs enrolling almost 2 500 post-stroke patients found the following, presented in Figure 4 below [7]:

Which are the best neurorehabilitation therapies available?
The short answer to this question is: it depends. Though, with ample scientific evidence, what can be generally said is that cardiorespiratory fitness training (e.g., walking) is one of the best things a person can do after a stroke [8]. Beyond that, the therapy needs to target the disability or impairment caused by the stroke with sufficient intensity and specificity.
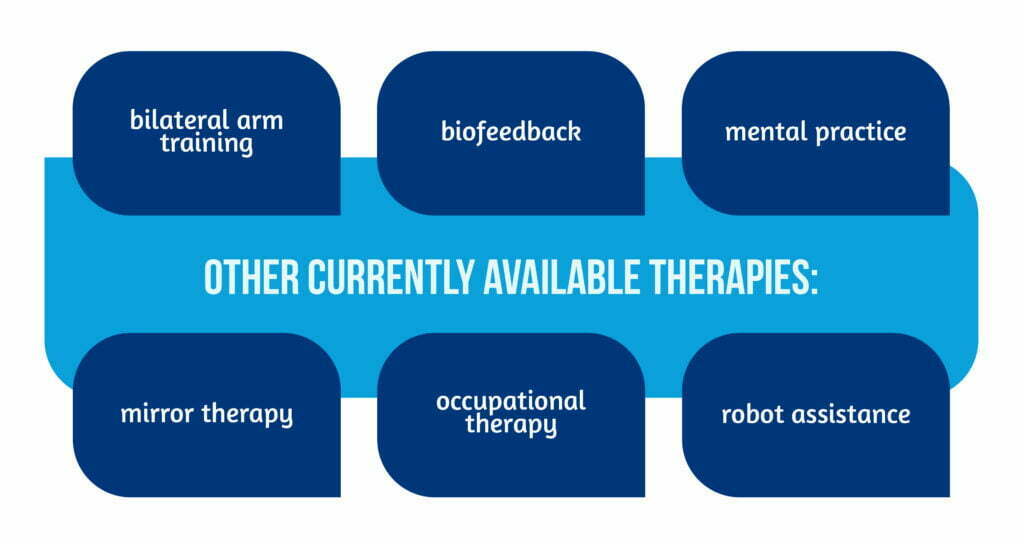
The methods featured here illustrate the diversity of approaches. Other currently available therapies [4] are described in Figure 5 below:
Some may not be available widely but it is worth exploring the “telerehabilitation” therapies offered remotely with minimal supervision to help stroke survivors recover from motor deficits, cognitive dysfunction, or depression from the comfort of their homes [9].
Conclusions
- Patients with motor neurological impairments may benefit more from rehabilitation following constraint-induced movement therapy and additional rigorous, experience-dependent learning.
- Constraint-induced language therapy and other techniques may help aphasic patients recover more quickly.
- Spatial neglect may be improved by prism adaptation therapy, virtual reality training, and therapies that implicitly integrate 3D motor and perceptual function.
- Transcranial magnetic stimulation (TMS) may cause a brain state known as permissiveness, which is beneficial in motor and cognitive rehabilitation.
- Transcranial direct current stimulation provides an appealing risk/cost profile for practicable future application and may enhance better mood and motor and cognitive rehabilitation outcomes [2].
References
- Maier M, Ballester BR, Verschure PFMJ. Principles of Neurorehabilitation After Stroke Based on Motor Learning and Brain Plasticity Mechanisms. Front Syst Neurosci 2019; 13:74; doi: 10.3389/fnsys.2019.00074.
- Barrett AM, Oh-Park M, Chen P, Ifejika NL. Neurorehabilitation: Five new things. Neurol Clin Pract 2013; 3(6):484-492; doi: 10.1212/01.CPJ.0000437088.98407.fa.
- Mehrholz J, Thomas S, Elsner B. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst Rev 2017; 8(8):CD002840; doi: 10.1002/14651858.CD002840.pub4.
- Corbetta D, Sirtori V, Castellini G, Moja L, Gatti R. Constraint-induced movement therapy for upper extremities in people with stroke. Cochrane Database Syst Rev 2015; 2015(10):CD004433. doi: 10.1002/14651858.CD004433.pub3.
- Zhang J, Yu J, Bao Y, Xie Q, et al. Constraint-induced aphasia therapy in post-stroke aphasia rehabilitation: A systematic review and meta-analysis of randomized controlled trials. PLoS One 2017; 12(8):e0183349; doi: 10.1371/journal.pone.0183349.
- Kim WJ, Rosselin C, Amatya B, Hafezi P, Khan F. Repetitive transcranial magnetic stimulation for management of post-stroke impairments: An overview of systematic reviews. J Rehabil Med 2020; 52(2):jrm00015. doi: 10.2340/16501977-2637.
- Laver KE, Lange B, George S, Deutsch JE, et al. Virtual reality for stroke rehabilitation. Cochrane Database Syst Rev 2017; 11(11):CD008349; doi: 10.1002/14651858.CD008349.pub4.
- Saunders DH, Sanderson M, Hayes S, Johnson L et al. Physical fitness training for stroke patients. Cochrane Database Syst Rev 2020; 3(3):CD003316; doi: 10.1002/14651858.CD003316.pub7.
- Sarfo FS, Ulasavets U, Opare-Sem OK, Ovbiagele B. Tele-Rehabilitation after Stroke: An Updated Systematic Review of the Literature. J Stroke Cerebrovasc Dis 2018; 27(9):2306-2318; doi: 10.1016/j.jstrokecerebrovasdis.2018.05.013.