Efficacy of mirror therapy in the recovery of severe hemiparesis after stroke

Keywords: stroke rehabilitation; upper limb rehabilitation; mirror therapy; motor recovery; hemineglect
Authors: Livia Popa, Oana Vanta
The applicability of Mirror Therapy in Rehabilitation
What is the efficacy of mirror therapy in the recovery of severe hemiparesis after stroke? One of the most important syndromes following a stroke is the paretic arm. The cortical motor representation is reduced due to a lack of use in the respective arm, with the sensory deficits limiting the cortical activation [1].
Rehabilitation strategies should be intensive, repetitive, and task-specific for neuroplasticity to lead to recovery after stroke.
The concept of mirror therapy has a neurophysiological foundation. A mirror is placed in the patient’s midsagittal plane, presenting the participant with the mirror image of their unaffected arm as if it were the paretic one. By moving the non-paretic upper extremity, the inversion of the visual image can obtain lateralized cortical activation [2].
In contrast to other therapies which require voluntary movement, mirror therapy (MT) can be implemented even in severely or completely paretic stroke survivors. MT uses visual stimuli to produce a desired response in the affected limb [3].
The research published by Dohle and collaborators in 2009 presents a therapy that can be applied in the early phase after stroke to improve motor, sensory, and attentional functions. The authors focused on evaluating the potential beneficial effect of mirror therapy on early recovery after a stroke [4].
Learn more about crossmodal illusions in neurorehabilitation and the effectiveness of intense neurorehabilitation of the upper limb after stroke.
Mirror therapy and brain activation
The involvement of different cortical regions in the processes that mediate recovery and the exact mechanism of MT remains unknown. However, the effects of MT are frequently linked to “mirror neurons“, neurons in both monkeys’ and humans’ premotor areas activated during observation of relevant movements [5].
Experimental data suggest the presence of a mirror neuron system devoted to mouth, hand, and foot actions in humans. A systematic activation of the observation–execution matching “mirror neuron” system of the parietal and premotor cortices can be used to affect modifications in these functions in ischemic stroke patients. Hand motor skills and speech production are strongly represented in these regions. The development of such skills played a vital evolutionary role; phylogenesis depended on observation–execution matching. Children’s development of such skills also depends on imitation, and good recovery from a stroke might also depend on using this system. The functional outcome of patients suffering from hand motor deficits can be influenced by tasks involving observation–execution matching [6].
Nonetheless, in Dohle’s studies, lateralized activations were reported in the occipital and posterior parietal regions rather than the premotor area in the only imaging experiment on inverted visual feedback [4, 7].
The precuneus region (area V6), as part of the neural network that supports self-representation in the mind, seems to play an important role [8].
Because of the observed body side, premotor areas may be active bilaterally without lateralization [9]. As a result, the therapeutic effect of MT may be transmitted by the visual illusion that behaviors performed by oneself are normal. This illusion will likely prevent or lessen the “learned non-use” of a paretic limb [10].
Mirror therapy for severe hemiparesis after stroke
Between October 2004 and April 2006, the authors [4] recruited 48 patients with severe hemiparesis after a first-ever ischemic stroke in the middle cerebral territory shorter than 8 weeks after the ischemic event.
Only thirty-six patients completed one of the two therapy protocols. The participants were divided into two equal groups: the mirror therapy (MT) and the control therapy (CT) groups [4]. Between the two groups, there was an imbalance regarding the number of activities of daily living (ADL); no differences could be established for demographic parameters. Moreover, the markers for patients’ cooperation and potential treatment (attention and vigilance during the study) were similar between MT and CT groups [4].
The patient eligibility criteria are highlighted in Figure 1 below [4].

The intervention protocol
The volunteers allocated to the MT group underwent 30-minute daily therapy sessions 5 days a week for 6 weeks, in addition to the standard therapy provided in the rehabilitation center (occupational therapy, physiotherapy, and ADL training). The participants from CT followed an equivalent treatment [4].
The sole difference between the two groups’ therapy protocols was the type of visual input they received and not the motor performance [4]. Patients observed a mirror image of the unaffected arm as if it were the affected one during MT (see Figure 2). No mirror was used during CT, and patients had a clear picture of the affected arm. All participants were reminded to move their affected limb “as well as possible”, during both therapy interventions, following Altschuler and associates’ initial approach [4, 11].
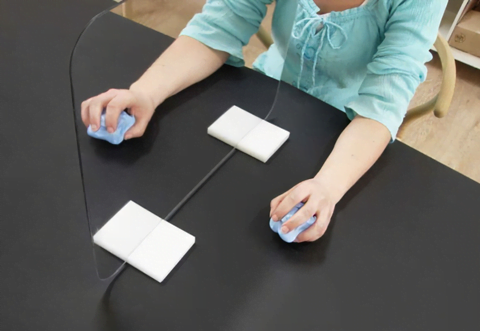
The major outcome measures were the upper limb Fugl-Meyer subscores, assessed by independent raters via videotape. Patients also underwent functional and neuropsychological testing [4].
Methods of evaluation and tools used in the study:
⦁ Fugl-Meyer test (7 upper limb subscores);
⦁ Action Research Arm Test;
⦁ Functional Independence Measure (FIM) – the motor part [4].
The Fugl-Meyer test has 63 items, scoring all major neurological symptoms on an ordinal scale from 0 to 2 (2 means no deficit). This test evaluates motor and non-motor functions: surface sensibility, proprioception, joint pain during passive movement, and range of motion.
The Action Research Arm test uses 4 subscales grasp, grip, pinch, and gross movement. The motor part of the FIM measures the performance in mobility and self-caring [4].
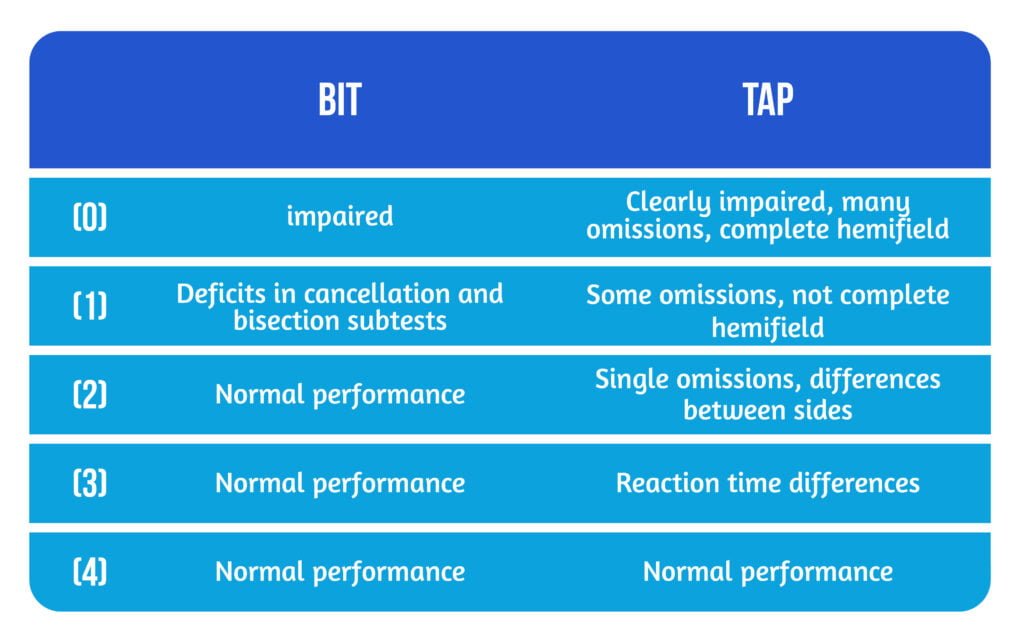
In order to assess the hemineglect, the study used several subtests of the Behavioral Inattention Test (BIT) and the test of attentional performance (TAP) with omissions and reaction times in each visual hemifield [4]. The possible scores are represented in Table 1.
Study results
There were improvements in both therapy groups in motor subscores, surface sensibility, and proprioception, with no adverse events or side effects noted in any of the two groups. The mean values improved in both groups because of the standard therapy and spontaneous recovery. Significant therapy effects were reported in favor of the MT group [4].
As for the motor function, the mean improvement of the MT group was 4.4 on the 14-point Fugl-Meyer subscale, while in the CT group, the mean improvement was 1.5. A beneficial effect of MT was regaining useful reach and grasp movements, evaluated with Action Research Arm Test.
Nonmotor symptoms improved significantly in the MT group. Regarding the improvement in surface sensibility (light touch), the study showed differences between the two groups. The mean improvement for the MT group was 0.8, while for CT patients were 0.2 on the 4-point Fugl-Meyer subscale.
At the beginning of the therapy, 20 of the 24 right-handed patients with right hemispheric lesions presented signs of hemineglect. The improvement of the neglect score was more significant in the MT group than in the CT group. For the level of ADL capacity (evaluated with the motor FIM), the study showed no difference between both therapy groups [4].
Conclusions
Mirror therapy supports the role of observing mirrored movement in cortical stimulation and promotes recovery after a severe stroke.
Observation of mirrored distal movements enhances cortical excitability, alike to actual movement execution. The effect is only present in distal arm muscles. Movement mirroring stimulates lateralized motor representations for the distal limb. Furthermore, the study showed that mirror therapy has a beneficial effect on hemineglect [4].
Movement observation also modulates cortical somatosensory representations. Viewing the stimulated arm enhances discrimination ability both in participants with brain damage and participants without brain affections. Adding passive movements of the affected limb to the MT can bring therapeutic value through bilateral arm training.
The work of Dohle et al. [4] showed relevant effects of MT on motor and sensory recovery, according to current neurophysiological findings. As mirror therapy can be easily used, patients can be instructed to practice it independently, at home, or even in the acute phase of stroke, promoting poststroke recovery.
References
- Altschuler EL, Wisdom SB, Stone L et al. Rehabilitation of hemiparesis after stroke with a mirror. Lancet. 1999;353(9169):2035-2036. DOI: 10.1016/s0140-6736(99)00920-4
- Fritz SL, Light KE, Patterson TS, Behrman AL, Davis SB. Active finger extension predicts outcomes after constraint-induced movement therapy for individuals with hemiparesis after stroke. Stroke. 2005;36(6): 1172-1177. DOI: 10.1161/01.STR.0000165922.96430.d0
- Gandhi DB, Sterba A, Khatter H, Pandian JD. Mirror Therapy in Stroke Rehabilitation: Current Perspectives. Ther Clin Risk Manag. 2020;16:75-85. doi:10.2147/TCRM.S206883
- Dohle C, Püllen J, Nakaten A, Küst J et al. Mirror therapy promotes recovery from severe hemiparesis: a randomized controlled trial. Neurorehabil Neural Repair. 2009;23(3):209-17. DOI: 10.1177/1545968308324786.
- Buccino G, Binkofski F, Fink GR, et al. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci. 2001;13(2):400-404. Available at: https://pubmed.ncbi.nlm.nih.gov/11168545/
- Small SL, Buccino G, Solodkin A. The mirror neuron system and treatment of stroke. Dev Psychobiol. 2012 Apr;54(3):293-310. DOI: 10.1002/dev.20504.
- Dohle C, Kleiser R, Seitz RJ, Freund HJ. Body scheme gates visual processing. J Neurophysiol. 2004;91(5):2376-2379. DOI: 10.1152/jn.00929.2003
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioral correlates. Brain. 2006;129:564-583. DOI: 10.1093/brain/awl004
- Shmuelof L, Zohary E. A mirror representation of others’ actions in the human anterior parietal cortex. J Neurosci. 2006;26(38):9736-9742 DOI: 10.1523/JNEUROSCI.1836-06.2006
- Ramachandran VS. Phantom limbs, neglect syndromes, repressed memories, and Freudian psychology. Int Rev Neurobiol. 1994;37:291-333. DOI: 10.1016/s0074-7742(08)60254-8
- Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The poststroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13-31. Available on: https://pubmed.ncbi.nlm.nih.gov/1135616/
- Available online: https://mrri.org/tag/mirror-therapy/