Recovery of motor function continues for more than one year after a stroke
Authors: Ioana Stanescu, Oana Vanta
Keywords: stroke rehabilitation, virtual reality training, neuroplasticity, upper limb rehabilitation, chronic stroke rehabilitation
Impact of stroke
Recovery of motor function continues for more than one year after a stroke! Stroke is the second cause of death and the first cause of long-term neurological disability worldwide. Disability occurs in half of the stroke survivors, and one-third of stroke patients are dependent on daily care activities [1]. Population ageing and successful treatments for acute stroke (thrombolysis and thrombectomy) could change the profile of stroke patients, decreasing mortality rates while increasing the disability rates of the survivors, as half of the stroke survivors have associated disabilities, and one-third lose autonomy. Rehabilitation is one of the most important interventions in stroke, aiming to improve patients’ disability and quality of life and decrease societal costs [2].
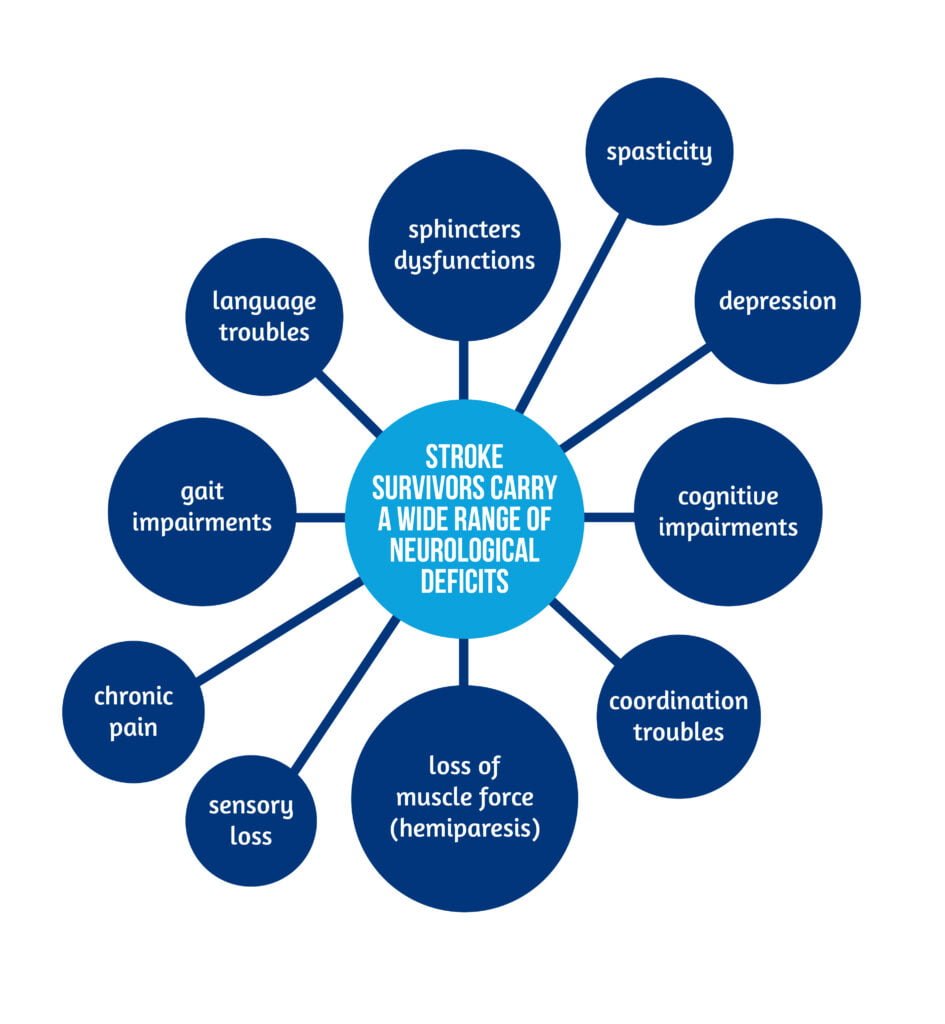
Stroke survivors carry a wide range of neurological deficits. as presented in Figure 1.
Learn more about Efficacy of mirror therapy in the recovery of severe hemiparesis after stroke and The Effectiveness of Robotic-Assisted Therapy in stroke rehabilitation.
Pathways in post-stroke motor recovery
Two main pathways in post-stroke motor recovery are behavioral restitution of motor control and compensation.
Behavioural restitution, also called “true recovery” refers to the return of a normal motor pattern of upper or lower limb movements to the level before the stroke. It is often incomplete and could be appropriately measured by changes in the Fugl-Meyer Assessment (FMA) scores. Enhanced neuroplasticity amplified by the effect of sensorimotor training is the underlying mechanism for the recovery of body function and structure [2–4].
Compensation refers to the ability to perform a motor task through a new strategy that uses less affected limb segments in a different manner. Compensatory strategies accomplished by learning are the background of this pathway as the patient learns new modalities to accomplish everyday tasks. The assessment uses scales measuring the ability to perform activities of daily living (ADLs): the Barthel Index (BI) or the Chedoke Arm and Hand Activity Inventory (CAHAI) [2–5].
The timeframe of recovery of motor function after a stroke
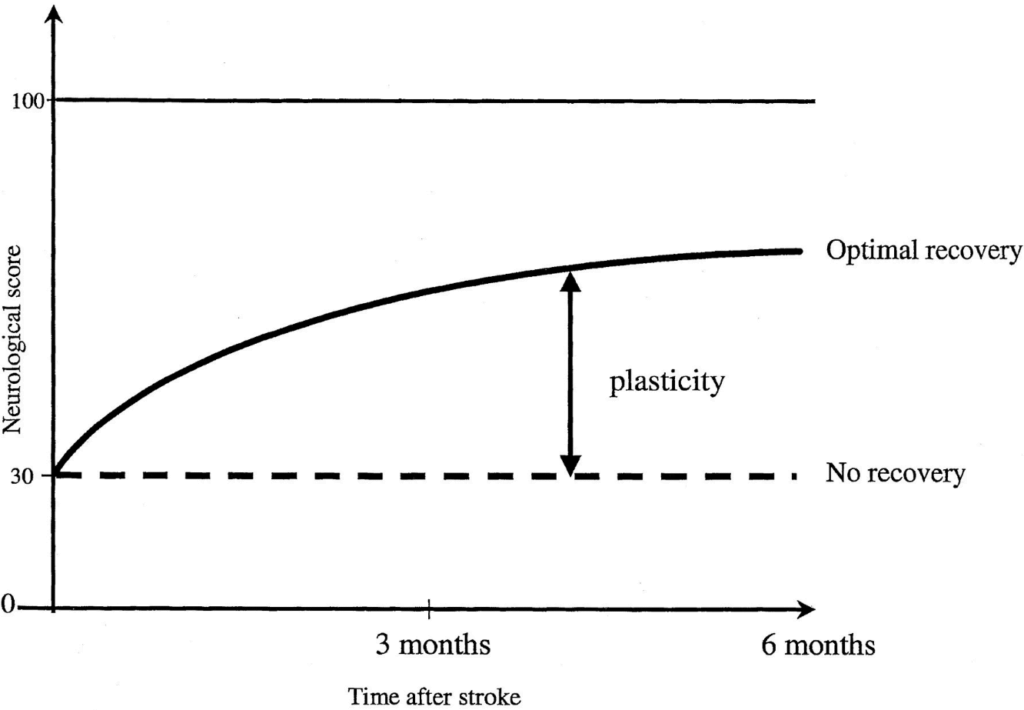
There is a critical period for recovery after a stroke that is considered to be the most sensitive for obtaining the most potent effects of treatment. This timeframe is called ‘the critical window for recovery” and is attributed to the first 3 to 6 months after the event. Post-stroke recovery has a non-linear pattern, with maximal spontaneous recovery in the first weeks after the stroke, followed by a plateau a few months after the onset, as shown in Figure 2.
Stroke evolution over time is divided into intervals, essential for implementing strategies for rehabilitation treatment:
- Acute stage: < 3 weeks from onset
- Subacute stage: 3 weeks – 6 months
- Early chronic stage: 6 months – 18 months
- Late chronic stage: more than 18 months from onset
It is widely accepted that the maximal recovery after a stroke occurs within the first 3 – 6 months after onset, in the subacute phase. After this “critical window of recovery”, the neurologic deficit becomes chronic and mainly stable.
A physiological background sustains this therapeutic window, with animal models and clinical studies demonstrating enhanced neuroplasticity in the first 3 to 6 months after a stroke [6–7] (see Figure 3).

The activity of neural repair mechanisms is maximal in the subacute stage and could be facilitated by treatments using sensorimotor stimulation [2]. The time window for recovery seems to be shorter in mild motor deficits (6, 5 weeks) and more prolonged in severe hemiplegia [8]. After that interval, there is a decrease in the responsiveness to treatment, and it has been suggested that physical therapy should be discontinued in the chronic stage post-stroke [2].
Rehabilitation on recovery of motor function in chronic stroke
Many trials have been conducted to establish the temporal evolution of stroke recovery in correlation with treatment responsiveness but without clear results. So far, the impact of rehabilitation on motor recovery in chronic stroke is not clearly established.
A study conducted by Ballester and collab in 2019 aimed to show that rehabilitation treatment improved the function of the affected upper limb during all stages of stroke evolution, including the chronic phase. The study included 219 patients with ischemic and hemorrhagic first-ever strokes and mild or moderate upper limb motor deficit (graded > 2 on the Medical Research Council scale for muscle force cotation). Among the included patients, 114 were in the chronic and late chronic stages after stroke.
Rehabilitation interventions in this study were occupational therapy (OT) and virtual reality (VR) based training using the Rehabilitation Gaming System (RGS). The RGS system components and functioning are shown in Figure 4.

The duration of intervention was heterogeneous: 3 weeks and 6 weeks, respectively, in 2 groups of chronic-stage patients and 3 to 4 weeks in late chronic-stage patients [2]. With measurements done at baseline and at the end of the intervention, the primary outcome was the improvement in the upper extremity (UE) motor activity, measured by the Upper Extremity section of the Fugl Meyer UE scale (UE-FM), and in the ability to perform daily activities (ADLs), measured by the CAHAI. Also, at the end of the intervention, autonomy was measured by changes in the BI.
Efficacy of VR gaming Systems in chronic stroke patients
In this study conducted by Ballester, VR training improved the motor function and the functional ability of the upper limb in stroke patients from both acute and subacute stages [2]. For the subacute group, benefits were maintained at 3 months follow-up. The sample of patients treated with OT in the chronic phase was too small to permit analysis.
The authors highlight that chronic stroke patients benefit after VR training, as demonstrated by improvements in UE-FM scores and CAHAI scores obtained at the end of the intervention. Patients in the subacute group had the most significant improvements among all groups. Those in early chronic stages performed better than patients in late chronic stages, showing an inverse correlation between the time since stroke onset and the improvement rate. Also, training duration was correlated to treatment benefits in a dose-dependent relationship. Rehabilitation treatment showed its effectiveness in both motor UE function and the ability to perform ADLs.
The authors outline the fact that there is sensitivity to rehabilitation treatment (VR training in this study) in the first 18 months after stroke. This effect is independent of the patient’s age and stroke severity at the onset stages [2]. However, treatment efficacy is clearly decreasing over time. The study results suggest that the period of increased neuroplasticity and neural repair (the therapeutic window for rehabilitation interventions) extends beyond one year after stroke. Ballister et al. postulate that in chronic stroke, compensatory mechanisms sustain the recovery of daily abilities [2].
Another strong point of Ballister’s study was assessing the minimal clinical important difference (MCID), which quantifies the changes in motor performances induced by rehabilitation interventions compared to the spontaneous evolution of affected functions after stroke. MCID threshold for upper limb motor function (UE-FM score) ranges from 16-30% in acute stages to 7-11% in early chronic stages. MCID threshold for UE abilities (CAHAI, Barthel Index) is 7% in the chronic phase [2, 9–10].
Conclusions on recovery of motor function
In stroke rehabilitation, there is a sensitive period in the first 3-6 months after injury where the neuroplasticity is enhanced and where improvements in the affected functions, possibly induced by rehabilitation interventions, are noticed. Beyond this “window for recovery”, rehabilitation treatment shows no or minimal benefit and should be discontinued.
The study by Ballester et al., which included stroke patients in the early chronic phase (6 to 18 months after stroke) and in the late chronic phase (more than 18 months), challenged this concept of the limited efficacy of rehabilitation treatment beyond 12 months. Virtual reality (VR) interventions were performed for extended periods (minimal 3 weeks, maximal 6 weeks), and motor performance in the affected upper limb (measured by UE-FM score) as well as the ability to perform daily living tasks (measured by CAHAI score and Barthel Index), were measured. Improvements in all scores were noticed at the end of the intervention, significant especially for early chronic patients. Other methods not described in this study also show efficiency in chronic stroke [2]. Overall, rehabilitation training with a VR gaming system is effective in chronic stroke patients.
The pattern of post-stroke recovery is maximal in the first 6 months, then fades out exponentially after 18 months. The authors postulated that there is still a period of sensitivity to treatment between 12 to 18 months after a stroke, which represents a reason why the window for rehabilitation interventions needs to be reassessed, and extended training continuation in chronic stroke patients should improve motor function and abilities.
References
- Markus HS. Reducing disability after stroke. International Journal of Stroke. 2022;17(3):249-250. doi:10.1177/17474930221080904
- Ballester BR, Maier M, Duff A, Cameirão M, Bermúdez S, Duarte E, Cuxart A, Rodríguez S, San Segundo Mozo RM, Verschure PFMJ. A critical time window for recovery extends beyond one-year post-stroke. J Neurophysiol. 2019 Jul 1;122(1):350-357. doi: 10.1152/jn.00762.2018.
- Bernhardt J, Hayward KS, Kwakkel G, et al. Agreed Definitions and a Shared Vision for New Standards in Stroke Recovery Research: The Stroke Recovery and Rehabilitation Roundtable Taskforce. Neurorehabilitation and Neural Repair. 2017;31(9):793-799. doi:10.1177/1545968317732668
- Kwakkel G, Lannin NA, Borschmann K, English C, Ali M, Churilov L, Saposnik G, Winstein C, van Wegen EEH, Wolf SL, Krakauer JW, Bernhardt J. Standardized measurement of sensorimotor recovery in stroke trials: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int J Stroke 12: 451– 461, 2017. doi:10. 1177/1747493017711813.
- Barreca SR, Stratford PW, Lambert CL, Masters LM, Streiner DL. Test-retest reliability, validity, and sensitivity of the Chedoke arm and hand activity inventory: a new measure of upper-limb function for survivors of stroke. Arch Phys Med Rehabil 86: 1616 –1622, 2005. doi:10.1016/j.apmr. 2005.03.017
- Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults: a review. Stroke. 2003 Jun;34(6):1553-66. doi: 10.1161/01.STR.0000071761.36075.A6.
- Ward NS. Restoring brain function after stroke: bridging the gap between animals and humans. Nat Rev Neurol 13: 244 –255, 2017, doi:10.1038/ nrneurol.2017.34.
- Hendricks HT, van Limbeek J, Geurts AC, Zwarts MJ. Motor recovery after stroke: a systematic review of the literature. Arch Phys Med Rehabil 83: 1629 –1637, 2002. doi:10.1053/apmr.2002.35473.
- Lang CE, Edwards DF, Birkenmeier RL, Dromerick AW. Estimating minimal clinically important differences of upper-extremity measures early after stroke. Arch Phys Med Rehabil 89: 1693–1700, 2008. doi:10.1016/j. apmr.2008.02.022
- Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther 92: 791–798, 2012. doi:10.2522/ptj. 20110009.