Post-stroke language impairments treatment using tDCS (Transcranial direct current stimulation)

Authors: Victor Dabala, Oana Vanta
Keywords: transcranial stimulation, electrical current, stimulation, aphasia, stroke, language deficits, anodal tDCS
Aphasia or post-stroke language impairments treatment using tDCS (Transcranial direct current stimulation)
Post-stroke impairments tend to be unpredictable, aphasia being no exception. Moreover, language disorders can evolve into a different phase without the proper intervention and intensity applied, with little to no deficit recovery. Frequently, the improvement can be surprisingly minimal. Different known therapies have been proposed, with behavioral speech therapy considered a pinnacle in rehabilitation. As far as transcranial direct current stimulation (tDCS) is involved, two approved methods are: anodal tDCS (A-tDCS) and sham tDCS (S-tDCS) [1].
To learn more about stroke rehabilitation, visit:
- Shedding light on Speech-Language Therapy: How stroke survivors can benefit from intensive rehabilitation training for chronic aphasia
- Accelerating the process of rehabilitation through virtual reality: The standing point of Reh@Task in post-stroke disability
Moreover, get more insight by watching the Interview with the president of the World Stroke Organization:
A more physical approach – Electrical stimulation
In a randomized control trial (RCT) by Fridriksson and al. [1], the authors investigated whether further research for anodal tDCS (used as an adjuvant intervention to classical behavioral speech therapy for the improvement of speech production – naming) can be considered futile in stroke survivors with chronic aphasia. The RCT implied a futility design, which demonstrates that for a particular treatment, further investigation should not be continued if that treatment method was demonstrated as inferior compared to others. Different papers approached the selected topic, expressing results favoring anodal tDCS [2, 3].
tDCS applies a low-intensity electrical stimulus (1-2 mA), traveling between two electrodes placed on the scalp of the subject. The procedure is non-invasive, and the safety issues regarding the entire intervention are limited. Nevertheless, additional research should be performed to explain the mechanism. What is presently confirmed is that as opposed to anodal stimulation (A-tDCS), which enhances neural integration, cathodal intervention (C-tDCS – a different type of cranial stimulation) is supposed to decrease brain activity [1].
Selecting the proper aphasic population
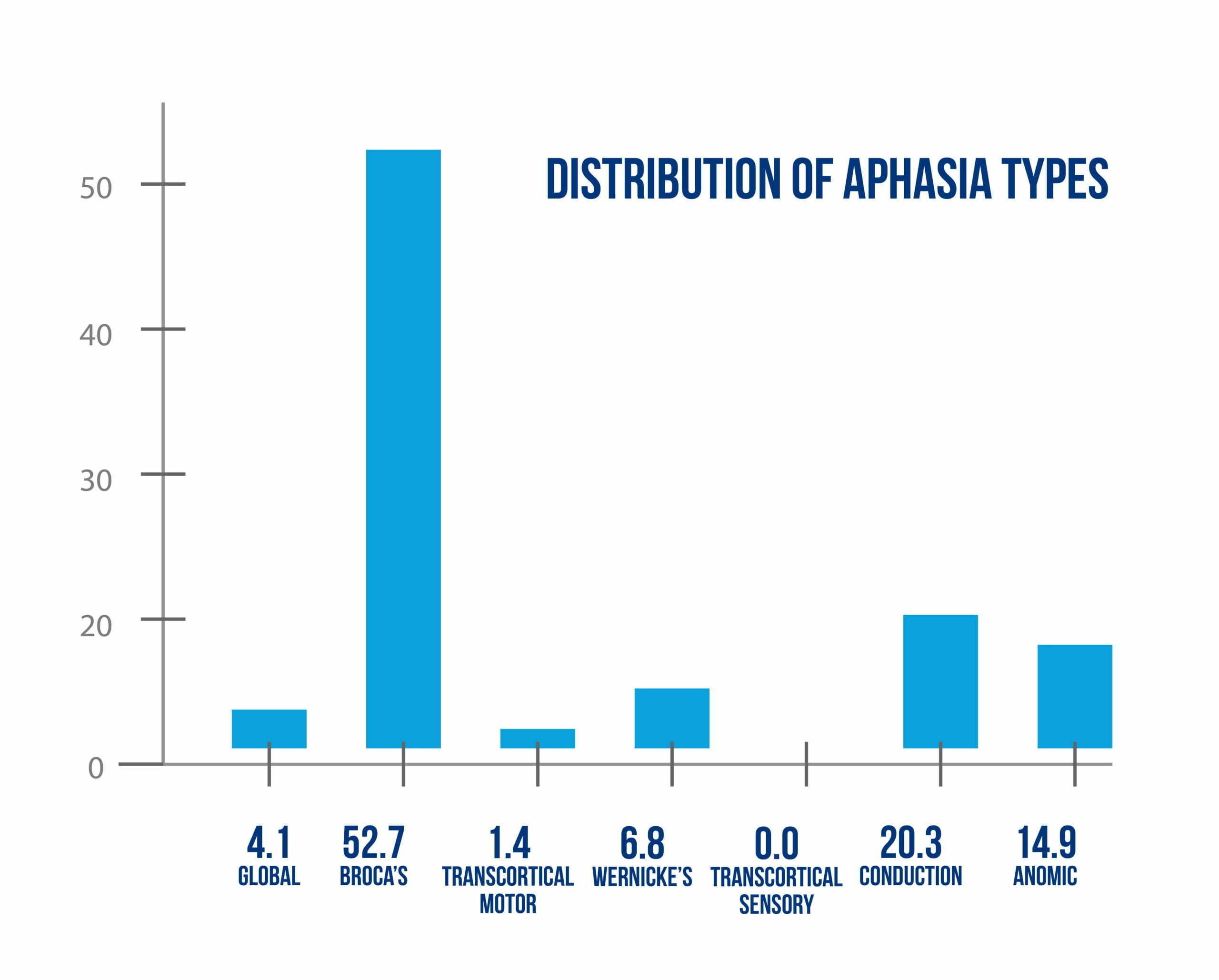
Over 5 years, 74 eligible patients (83% of all the identified subjects) were enrolled in the anodal tDCS RCT by Fridriksson and al. with different types of aphasia (Figure 1).
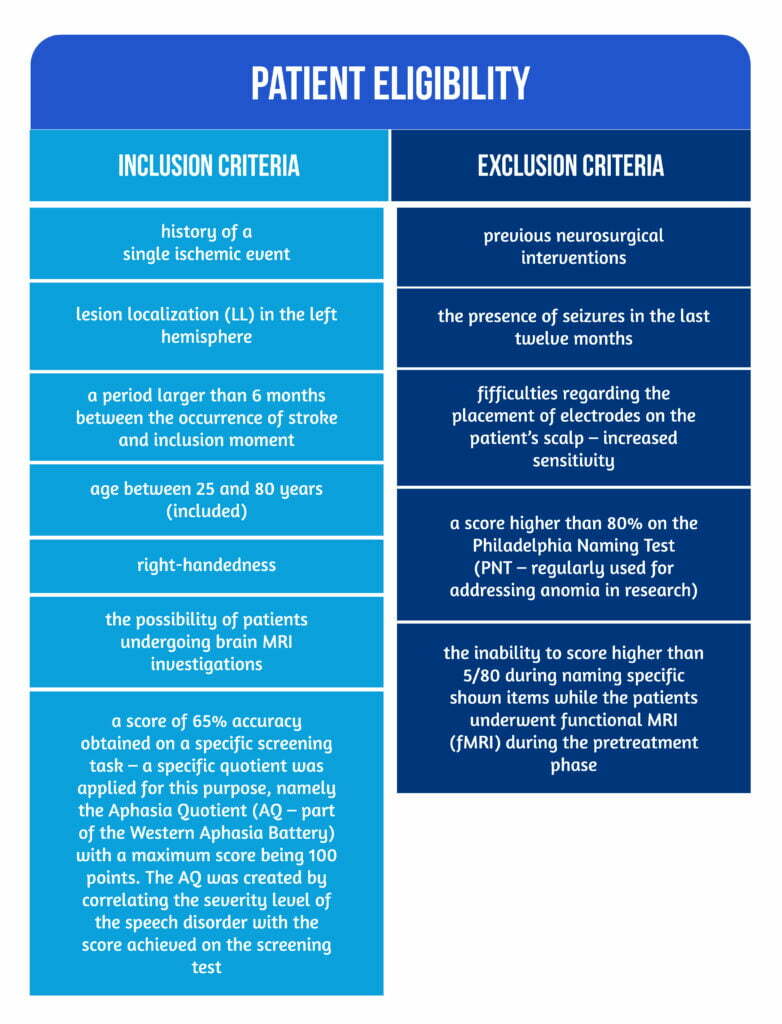
The patient eligibility criteria are highlighted in Figure 2 below.
Patients were randomly assigned to one of two groups (A-tDCS / S-tDCS) using a unique algorithm. The transcranial stimulation was treated as an adjuvant intervention coupled with anomia behavioral interventions. The anodal transcranial direct stimulation group consisted of 34 patients, compared to a larger sham-tDCS group (40 patients). All study participants (including the research team) were blinded during the repartition [1].
Two days were assigned for the initial visit. Evaluations for the neurological, cognitive, and linguistic status were performed, as well as the recording of each patient’s medical history.
The outcomes used in the RCT were evaluated using:
1) The National Institutes of Health Stroke Scale (NIHSS),
2) The Boston Naming Test–Second Edition (BNT),
3) The Apraxia of Speech Rating Scale (ASRS) – from the Apraxia Battery for Adults-Second Edition (ABA-2), 4) WAB-R,
5) The Pyramids and Palm Trees Test (PPTT),
6) The matrix reasoning subtest of the Wechsler Adult Intelligence Scale, Third Edition (WAIS) [1].
Current medications or other known therapies of the subjects were recorded subsequently [1].
Anode and cathode – Exploring the intervention
The intensity of the A-tDCS electrical current was set to 1 mA, generated by a Phoresor II stimulator. The selection of 1 mA is considered less likely to induce lesions than other intensities (e.g., 2 mA). The two electrodes were placed as follows: the anode on the cortical area that was assessed (the temporal region expressed the highest activation on fMRI during the pre-treatment naming sessions; therefore, it was selected), while the cathode was located on the scalp corresponding to the frontal region on the other side, superior to the right eyebrow. A latex cap was placed on the scalp of each patient. After positioning the anode electrode, the cap had to be taken out, maintaining the electrodes in position using adhesive bandages [1].
During the first 20 minutes of behavioral therapy, electrical stimulation using the anode current was applied. Based on previous research [4], the 20 minutes block can be easily tolerated by patients without reporting severe adverse effects (SAE). In addition, because sensations like itching or tingling can be associated with the first few moments after the start of the simulation, a similar physical impression was induced in patients undergoing S-tDCS [1].
The intervention consisted in showing the patients a virtual interface, presenting representations of familial objects or beings (e.g., a cat) for two seconds. The respective depictions had to be associated with words heard by the patients via headphones (with a speaker pronouncing the words in real-time). Two responses could be recorded based on the link between the spoken words and the image (green and red buttons for positive, respectively, negative correlations). The lower part of the speaker’s face could be seen on the interface. Around 50% of the image-words pairs shown were positive matches [1].
Each group underwent daily 45-minute sessions of computerized treatment over the course of 3 weeks. After each behavioral treatment session, a complete assessment for vital signs, possible adverse effects (AE), and additional discomfort was completed. The Wong-Baker FACES Pain Rating Scale (WBF) was used for the latter. The most common discomfort was the presence of tingling shortly after the A-tDC stimulation started. Two subjects in the anodal tDCS population experienced a local erythema/redness against no patients in the S-tDCS, which resolved after two days. A score of 3 on the Wong-Baker FACES Pain Rating Scale was registered for two patients, corresponding to ‘’hurts ever more”. The respective score was recorded four times in both patients. It can be noted that given the fact that over 500 sessions were applied in the two groups, the incidence of adverse effects would be considered ‘’very low”. Previous research data support the statement that tDCS is not associated with severe AEs [1, 5].
Lessons from the trial
No significant differences between the patient’s demographic data and clinical characteristics could be found in the two groups (For the clinical status variable, the values were higher at baseline in the anodal stimulation group). Compared to a previous large cohort study (AphasiaBank) in terms of severity and aphasia types, the randomized controlled trial by Fridriksson and al. included more severe patients based on the AQ while presenting a larger proportion of patients with Broca aphasia (39 subjects) [1].
Comparing the last rehabilitation session accuracy against the first one, 73 out of the 74 patients improved on the computerized task. One subject from each group did not complete all the sessions (14/15 for both of them). The anodal tDCS patient was considered a drop-out after the eleventh session, while the s-tDCS patient suffered a seizure and, therefore, the electrical stimulation was stopped further on. Regarding adverse effects, 8 non-serious AEs were registered, with no significant differences regarding the number of AEs [1, 6].
Anomia (naming) was considered the principal outcome of the RCT because of its prevalence (as a part of all types of aphasia / individual disorder) and the importance of being capable of retrieving words. Furthermore, the quality of life (QoL) is affected in patients with anomia, as naming is a vital part of the daily-performed activities. However, despite this statement, the randomized controlled trial results cannot prove that an improvement in naming can be correlated with an improvement in the QoL [1].
The A-tDCS had a better outcome than S-tDCS, indicating the necessity of a larger trial for assessing the effects of anodal stimulation on behavioral treatment. In addition, the baseline differences between the two studied populations were approximately twice as large in the anodal stimulation group compared to the sham stimulation [1].
A failure to reject the null hypothesis was found, which stated that further study of A-tDCS is futile, showing that anodal tDCS applied as an adjuvant in anomia treatment is feasible, with the recommendation of being furtherly assessed. Furthermore, interventions for which futility could not be demonstrated are encouraged to be further investigated in different research contexts (e.g., RCTs focusing on the superiority of one intervention or systematic reviews) [1].
References
- Fridriksson J, Rorden C, Elm J, Sen S, et al. Transcranial Direct Current Stimulation vs. Sham Stimulation to Treat Aphasia After Stroke: A Randomized Clinical Trial. JAMA Neurol. 2018;75(12):1470-1476. doi: 10.1001/jamaneurol.2018.2287.
- Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. 2010;41(6):1229-1236. doi:f 10.1161/STROKEAHA.109.576785
- Kang EK, Kim YK, Sohn HM, Cohen LG, Paik N-J. Improved picture naming in aphasia patients treated with cathodal tDCS to inhibit the right Broca’s homologue area. Restor Neurol Neurosci. 2011;29(3):141-152. DOI: 10.3233/RNN-2011-0587
- Nitsche MA, Cohen LG, Wassermann EM, et al.. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1(3):206-223. doi: 10.1016/j.brs.2008.06.004
- Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving aphasia in patients with aphasia after stroke. Cochrane Database Syst Rev. 2015;(5):CD009760. DOI: 10.1002/14651858.CD009760.pub4
- Macwhinney B, Fromm D, Forbes M, Holland A. AphasiaBank: Methods for studying discourse. Aphasiology. 2011;25(11):1286-1307. doi: 10.1080/02687038.2011.589893